REVIEW ARTICLE
Scandinavian SSAI clinical practice guideline on choice of inotropic agent for patients with acute circulatory failure
M. H. Møller1 , A. Granholm1 , E. Junttila2 , M. Haney3 , A. Oscarsson-Tibblin4 , A. Haavind5 , J. H. Laake6 , E. Wilkman7 , K. O. Sverrisson € 8 and A. Perner1
1 Department of Intensive Care 4131, Copenhagen University Hospital, Rigshospitalet, Copenhagen, Denmark
2 Department of Anaesthesiology, Tampere University Hospital, Tampere, Finland
3 Anaesthesiology and Intensive Care Medicine, Umea University, Umea, Sweden
4 Department of Anaesthesiology and Intensive Care, Department of Medicine and Health, Linkoping University, Link € oping, Sweden €
5 Department of Anaesthesiology and Intensive Care, University Hospital Northern Norway, Tromsø, Norway
6 Division of Critical Care, Oslo University Hospital, Oslo, Norway
7 Division of Intensive Care Medicine, Department of Perioperative, Intensive Care and Pain Medicine, Helsinki University Hospital, University of Helsinki, Helsinki, Finland
8 Department of Anesthesia & Critical Care, Landspitali University Hospital of Iceland, Reykjavik, Iceland
Correspondence
M. H. Møller, Department of Intensive Care
4131, Copenhagen University Hospital
Rigshospitalet, Blegdamsvej 9, DK – 2100
Copenhagen, Denmark
E-mail: mortenhylander@gmail.com
Conflict of interest
The authors declare no relevant conflicts of interest.
Funding
This guideline was initiated and supported by the Scandinavian Society of Anaesthesiology and Intensive Care Medicine (SSAI).
Submitted 28 December 2017; accepted 3 January 2018; submission 24 November 2017.
Citation
Møller MH, Granholm A, Junttila E, Haney M, Oscarsson-Tibblin A, Haavind A, Laake JH, Wilkman E, Orn Sverrisson K, Perner A. € Scandinavian SSAI clinical practice guideline on choice of inotropic agent for patients with acute circulatory failure. Acta Anaesthesiologica Scandinavica 2018
doi: 10.1111/aas.13089
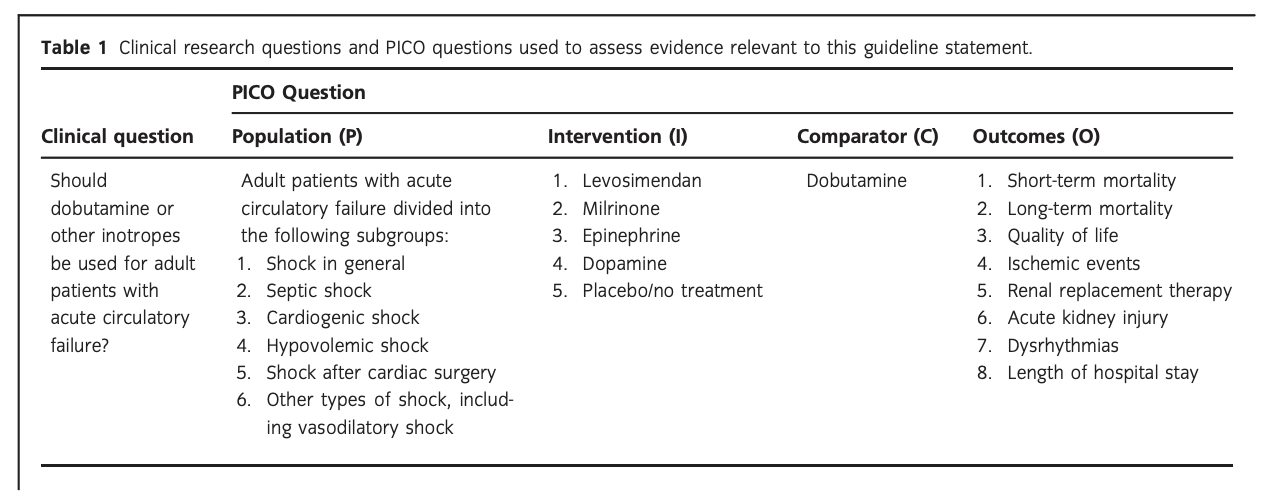
Background: Adult critically ill patients often suffer from acute circulatory failure and those with low cardiac output may be treated with inotropic agents. The aim of this Scandinavian Society of Anaesthesiology and Intensive Care Medicine guideline was to present patient-important treatment recommendations on this topic.
Methods: This guideline was developed according to GRADE. We assessed the following subpopulations of patients with shock: (1) shock in general, (2) septic shock, (3) cardiogenic shock, (4) hypovolemic shock, (5) shock after cardiac surgery, and (6) other types of shock, including vasodilatory shock. We assessed patient-important outcome measures, including mortality and serious adverse reactions.
Results: For all patients, we suggest against the routine use of any inotropic agent, including dobutamine, as compared to placebo/no treatment (very low quality of evidence). For patients with shock in general, and in those with septic and other types of shock, we suggest using dobutamine rather than levosimendan or epinephrine (very low quality of evidence). For patients with cardiogenic shock and in those with shock after cardiac surgery, we suggest using dobutamine rather than milrinone (very low quality of evidence). For the other clinical questions, we refrained from giving any recommendations or suggestions.
Conclusions: We suggest against the routine use of any inotropic agent in adult patients with shock. If used, we suggest using dobutamine rather than other inotropic agents for the majority of patients, however, the quality of evidence was very low, implying high uncertainty on the balance between the benefits and harms of inotropic agents.
Editorial comment
Failure to generate sufficient cardiac output is a challenge in patients with acute circulatory failure. This guideline analyzes the available evidence for increasing inotropy, which is scarce. It concludes that no agent should be used on a routine basis, while dobutamine emerges as the drug of choice when applied with caution to specific patient groups.
Acta Anaesthesiologica Scandinavica 62 (2018) 420–450
© 2018 The Authors. Acta Anaesthesiologica Scandinavica published by John Wiley & Sons Ltd on behalf of Acta Anaesthesiologica Scandinavica Foundation
This is an open access article under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs License, which permits use and 420 distribution in any medium, provided the original work is properly cited, the use is non-commercial and no modifications or adaptations are made.

Acute circulatory failure or shock is a life-threatening condition that needs prompt and adequate treatment, as it may progress to organ failure and death. Shock is a common condition in critical care medicine, affecting about one third of patients in the intensive care unit (ICU).1
Resuscitation of patients in shock must be early and appropriate to prevent or limit vital organ injury. Initial support of the failing circulation usually includes fluid resuscitation in combination with the administration of a vasopressor.1 In two recently published Scandinavian Society of Anaesthesiology and Intensive Care Medicine (SSAI) clinical practice guidelines, we have proposed recommendations regarding choice of fluid2 and choice of first-line vasopressor3 in the management of adult patients with acute circulatory failure. In collaboration with the Canadian Critical Care Society, SSAI has also recently issued recommendations for blood pressure targets in adult critically ill patients with hypotension.4
Subsets of patients with shock, including patients with heart failure may, however, not respond adequately to volume expansion and vasopressors, and additional support, including administration of inotropic agents may be required to restore cardiac output and organ perfusion. Inotropic agents commonly used include the synthetic catecholamine dobutamine, the endogenous catecholamine epinephrine, the phosphodiesterase III inhibitor milrinone, and the calcium sensitizer levosimendan.5 Furthermore, dopamine possesses inotropic properties, and is sometimes used as an inotropic agent.6
The Clinical Practice Committee of the SSAI initiated this guideline on choice of inotropic agent in adult patients with acute circulatory failure. The aim was to summarize the available evidence and provide recommendations according to current standards for trustworthy guidelines.7–9
Methods
Process
The Clinical Practice Committee of SSAI appointed national members of the guideline task force for Acute Circulatory Failure (the authors of this study). This group identified four key interventions needing guidelines, including fluid resuscitation,2 vasopressor therapy,3 inotropic therapy, and cardiovascular diagnostics and monitoring. This is the group’s third guideline: choice of inotropic agent for adult patients with acute circulatory failure.
We have prepared this guideline according to the AGREE statement.10
Clinical question
‘Which inotropic agent should be used for adult critically ill patients with acute circulatory failure?’
Population
The population of interest was adult patients (as defined in the original trials) with acute circulatory failure/shock (as defined in the original trials) receiving inotropes in a high-dependency setting in hospital, including the emergency department, ICU, operating room, and recovery room. The following subpopulations were assessed: patients with (1) shock in general (any type of shock), (2) septic shock, (3) cardiogenic shock, (4) hypovolemic shock, (5) shock after cardiac surgery, and (6) other types of shock, including vasodilatory shock. Acute circulatory failure and shock are used interchangeably throughout this guideline, and were defined as inadequate/hypoperfusion of tissue and organs.
Intervention(s)
We assessed any dose of the following inotropes:
(1) levosimendan, (2) milrinone, (3) epinephrine, (4) dopamine, and (5) placebo/no treatment. We defined inotropic agents as drugs with positive inotropic effect leading to increased stroke volume and cardiac output.

Acta Anaesthesiologica Scandinavica 62 (2018) 420–450
© 2018 The Authors. Acta Anaesthesiologica Scandinavica published by John Wiley & Sons Ltd on behalf of Acta Anaesthesiologica Scandinavica Foundation.
Comparator
The control inotropic agent was dobutamine (any dose).
We expected dobutamine to be the most widely studied drug, and thus chose dobutamine as the comparator and the other inotropes as experimental interventions.
Outcome(s)
The following patient-important outcome measures11 were assessed at the time of longest follow-up:
Critical outcomes
- Short-term mortality (0–90 days, including in-ICU and in-hospital mortality)
- Long-term mortality (more than 90 days)
- Quality of life as defined in the included trials Important outcomes
- Ischemic events as defined in the included trials
- Use of renal replacement therapy
- Acute kidney injury as defined in the included trials
- Dysrhythmias as defined in the included trials
- Hospital length-of-stay (LOS)
We excluded systematic reviews and trials done in children, those assessing prophylactic use of inotropes, those not reporting the predefined patient-important outcome measures, and those not comparing dobutamine vs. another inotropic agent, including those comparing combinations of inotropes or head-to-head comparison of other inotropes than dobutamine. Systematic reviews and trials allowing the use of adjuvant vasopressors were not excluded if the vasopressor used was identical in both arms. Cross-over trials and trials in which patients were systematically treated with either the intervention or comparator drug prior to or after randomization were also excluded. Search strategy We systematically searched PubMed (January 1966 to 25 September 2017), Cochrane Library (Issue 4, September 2017), and Epistemonikos.
Search strategy
We systematically searched PubMed (January 1966 to 25 September 2017), Cochrane Library (Issue 4, September 2017), and Epistemonikos for systematic reviews of randomized clinical trials (RCTs) and RCTs comparing dobutamine with other inotropic agents on 25 September 2017. No language restriction was employed. We used the following search strategies:
- PubMed: (dobutamine OR inotrope* OR inodilat*) AND (levosimendan OR milrinone OR epinephrine OR dopamine OR placebo OR ‘control’ OR ‘no treatment’) AND (shock OR cardiac OR ‘heart failure’). Filters: ‘Randomized controlled trials’ ‘Systematic reviews’; and ‘Meta-analyses’.
- Cochrane Library: ‘shock’ using the ‘Cochrane Review’ filter.
- Epistemonikos: same search as for PubMed adapted and without filters.
Statistics and GRADE
Specific clinical questions were formulated using the relevant patient population and/or clinical problem (P), the intervention (I) under scrutiny, the comparator (C), and the predefined patient-important outcomes (O)12 – PICO questions (Table 1). Mantel-Haenszel statistics and random effects models were used to generate summary estimates/meta-analyses (Review Manager Version 5.3, The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). We used trial sequential analysis (TSA) to assess the risk of random errors (spurious findings) due to repetitive testing and sparse data13. TSA was applied using an a priori 20% relative risk reduction, an alfa of 5%, beta of 90%, and a control event proportion according to the results from the included trials. TSA-adjusted 95% confidence intervals (CIs) were estimated (Appendix S1) and are reported in the summary of finding tables (Appendix S2). If less than 5% of the required information size had been accrued, no TSA could be conducted. We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system for formulating clinical questions, assessing the quality of evidence, generating anticipated absolute effects, and for moving from evidence to recommendations.9 In brief, we downgraded the quality of evidence (our confidence in the effect estimates) for an intervention for identified risks of bias (including baseline imbalance, lack of blinding, academic/financial conflicts of interest, or early termination of trials), inconsistency (unexplained heterogeneity), indirectness (including extrapolation from other patient populations or use of surrogate outcomes), imprecision (wide confidence interval around the effect estimate), or publication bias. Accordingly, the quality of evidence was rated from ‘high’ to ‘very low’. We used GradePro v. 3.5 to prepare summary of finding tables with anticipated relative and absolute effects for the outcomes, together with our confidence in the effect estimates (Appendix S2). When moving from evidence to recommendations, four factors were considered and integrated: benefits and harms, quality of evidence, values and preferences (of patients or their proxies), and cost considerations. GRADE classifies recommendations as ‘strong’ when virtually all informed patients would choose the recommended management strategy. ‘Weak’ recommendations apply when fully informed patients would choose different management strategies, and reflects a close call between benefits and harms, uncertainty regarding treatment effects, questionable cost effectiveness, or variability in values and preferences.9,14 The author group agreed upon all the recommendations in this guideline. Strong recommendations were given the wording ‘we recommend’, and weak recommendations ‘we suggest’. We followed standards for trustworthy guidelines through use of the GRADE system, management of intellectual and financial conflicts of interest on a recommendation per recommendation basis (Appendix S3), a peer review process, and a plan for updating of recommendations. We did not include patient representatives in the guideline process.
Results
The results and recommendations based on the PICOs are presented below, in Table 2, and in the summary of finding tables given in Appendix S2.
A. Dobutamine vs. other inotropes in patients with shock in general

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared the use of dobutamine with that of levosimendan in patients with shock in general (Fig. 1, Table S1A in Appendix S2). In reference to our recommendation for patients with septic shock, we suggest using dobutamine (extrapolation).
The quality of evidence was downgraded due to risk of bias, indirectness, and imprecision.
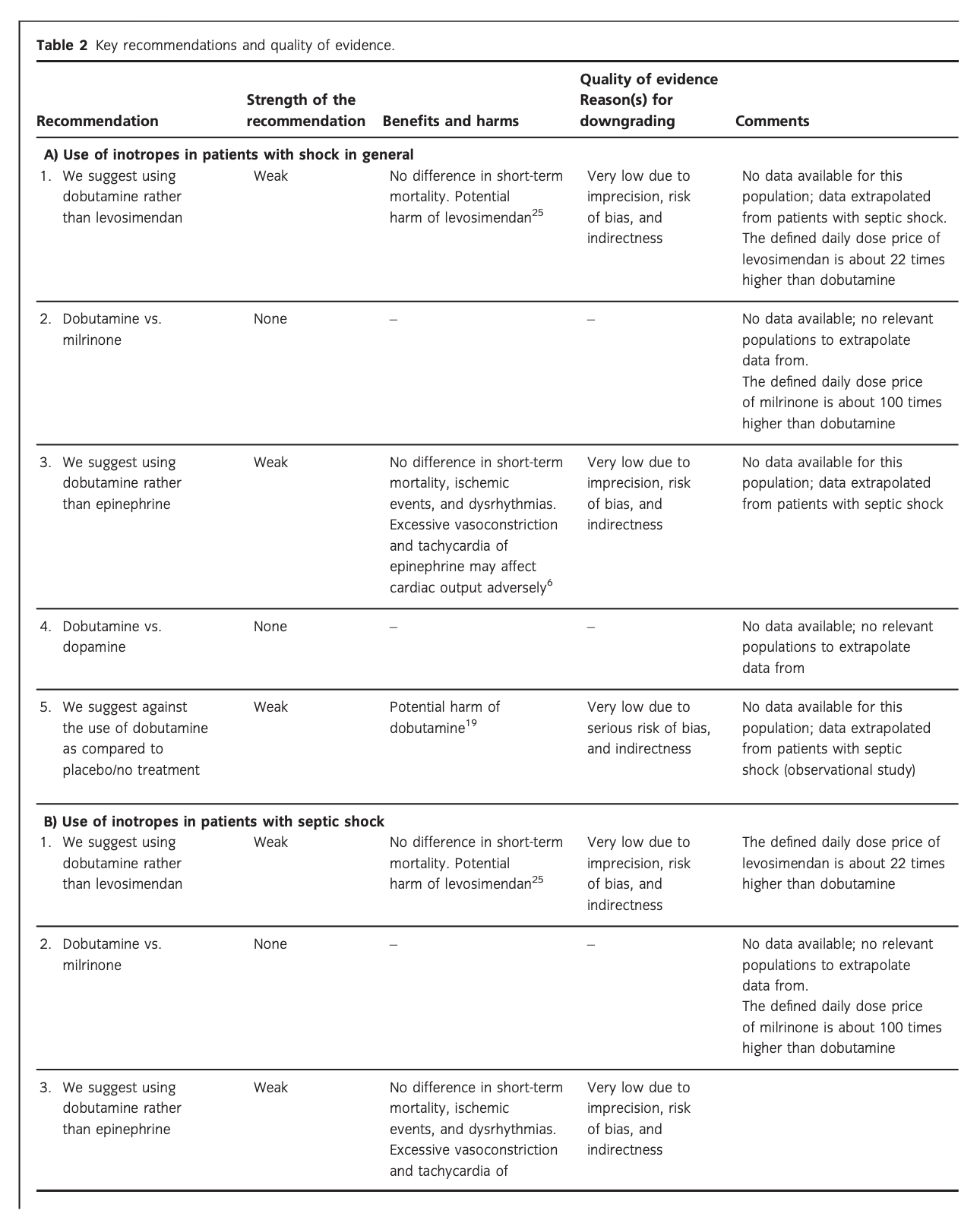

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared the use of dobutamine with that of milrinone in patients with shock in general (Fig. 1, Table S1B in Appendix S2). We refrain from giving any recommendations or suggestions on using dobutamine vs. milrinone for patients with shock in general, due to the lack of data and no relevant populations to extrapolate from. Importantly, we recommend that if clinicians prefer to use milrinone rather than dobutamine in this population, they do so in the context of high-quality RCTs, given the lack of data on the balance between the benefits and harms of milrinone in patients with acute circulatory failure in general.15 Of note, the defined daily dose price of milrinone is about 100 times higher than that of dobutamine.16

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared the use of dobutamine with that of epinephrine in patients with shock in general (Fig. 1, Table S1C in Appendix S2). In reference to our recommendation for patients with septic shock, we suggest using dobutamine (extrapolation).
The quality of evidence was downgraded due to imprecision, risk of bias, and indirectness.


No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared the use of dobutamine with that of placebo/no treatment in patients with shock in general (Fig. 1, Table S1E in Appendix S2). Importantly, potential harm of dobutamine has been suggested in a propensity-matched observational study in patients with septic shock.19 In reference to our recommendation for patients with septic shock, we suggest against routine use of dobutamine (extrapolation).
The quality of evidence was downgraded due to serious risk of bias and indirectness.
A Short-term mortality
No data.
B Long-term mortality
No data.
C Quality of life
No data.
D Ischemic events
No data.
E Renal replacement therapy
No data.
F Acute kidney injury
No data.
G Dysrhythmias
No data.
H Hospital length-of-stay
No data.
Fig. 1. Forest plot of (A) short-term mortality, (B) long-term mortality, (C) quality of life, (D) ischemic events, (E) renal replacement therapy, (F) acute kidney injury, (G) dysrhythmias, and (H) hospital length-ofstay in randomized trials of dobutamine vs. other inotropes for patients with shock in general.
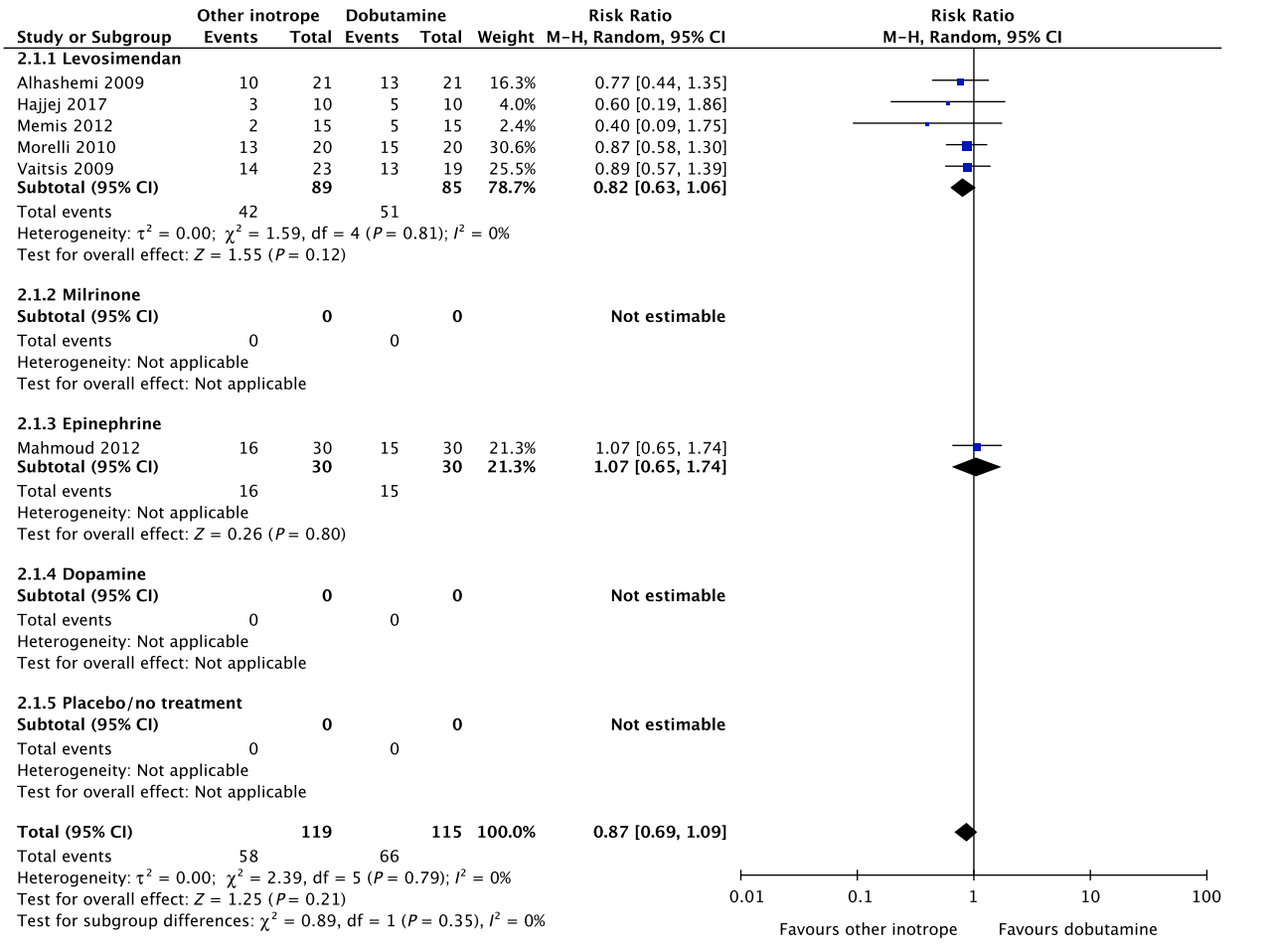
B. Dobutamine vs. other inotropes in patients with septic shock

In an updated meta-analysis comprising five trials,20–24 we found no statistically significant difference in short-term mortality in patients with septic shock treated with dobutamine vs. levosimendan (Fig. 2, Fig. S1A in Appendix S1; Table S2A in Appendix S2). None of the other predefined patient-important outcome measures have been assessed. In the recently published LEOPARDS trial, in which adult patients with sepsis were randomized to levosimendan or placebo, levosimendan was associated with a lower likelihood of successful weaning from mechanical ventilation and a higher rate of supraventricular tachyarrhythmia compared to placebo.25 This should caution the use of levosimendan in patients with sepsis, which is why we suggest using dobutamine rather than levosimendan in patients with septic shock. Of note, the defined daily dose price of levosimendan is about 22 times higher than that of dobutamine.16
The quality of evidence was downgraded due to risk of bias, indirectness, and imprecision.

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared the use of dobutamine with that of milrinone in patients with septic shock (Fig. 2, Table S2B in Appendix S2). We refrain from giving any recommendations or suggestions on using dobutamine or milrinone for patients with septic shock, due to the lack of data and no relevant populations to extrapolate from. Importantly, we recommend that if clinicians prefer to use milrinone rather than dobutamine in this population, they do so in the context of high-quality RCTs, given the lack of data on the balance between benefits and harms of milrinone in patients with acute circulatory failure in general.15 Of note, the defined daily dose price of milrinone is about 100 times higher than that of dobutamine.16

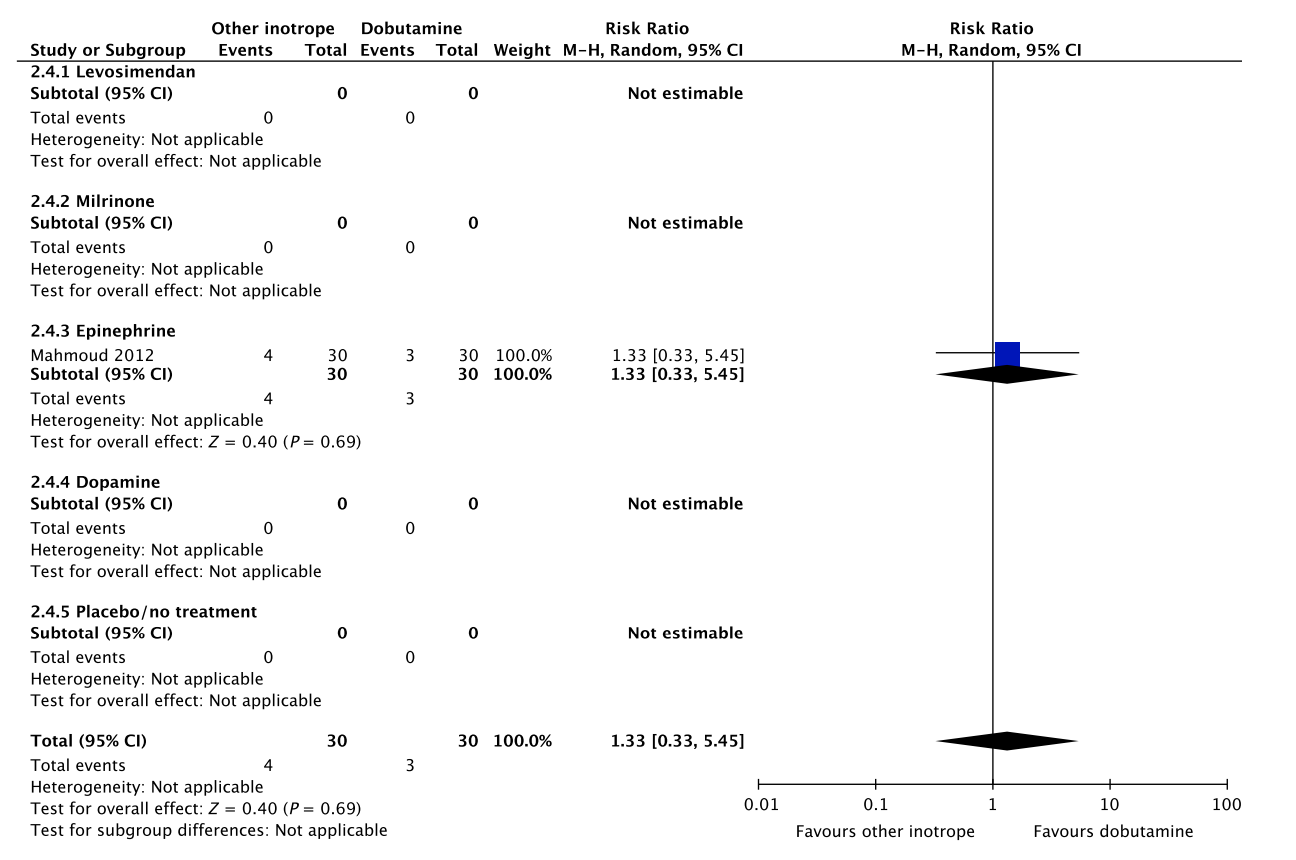
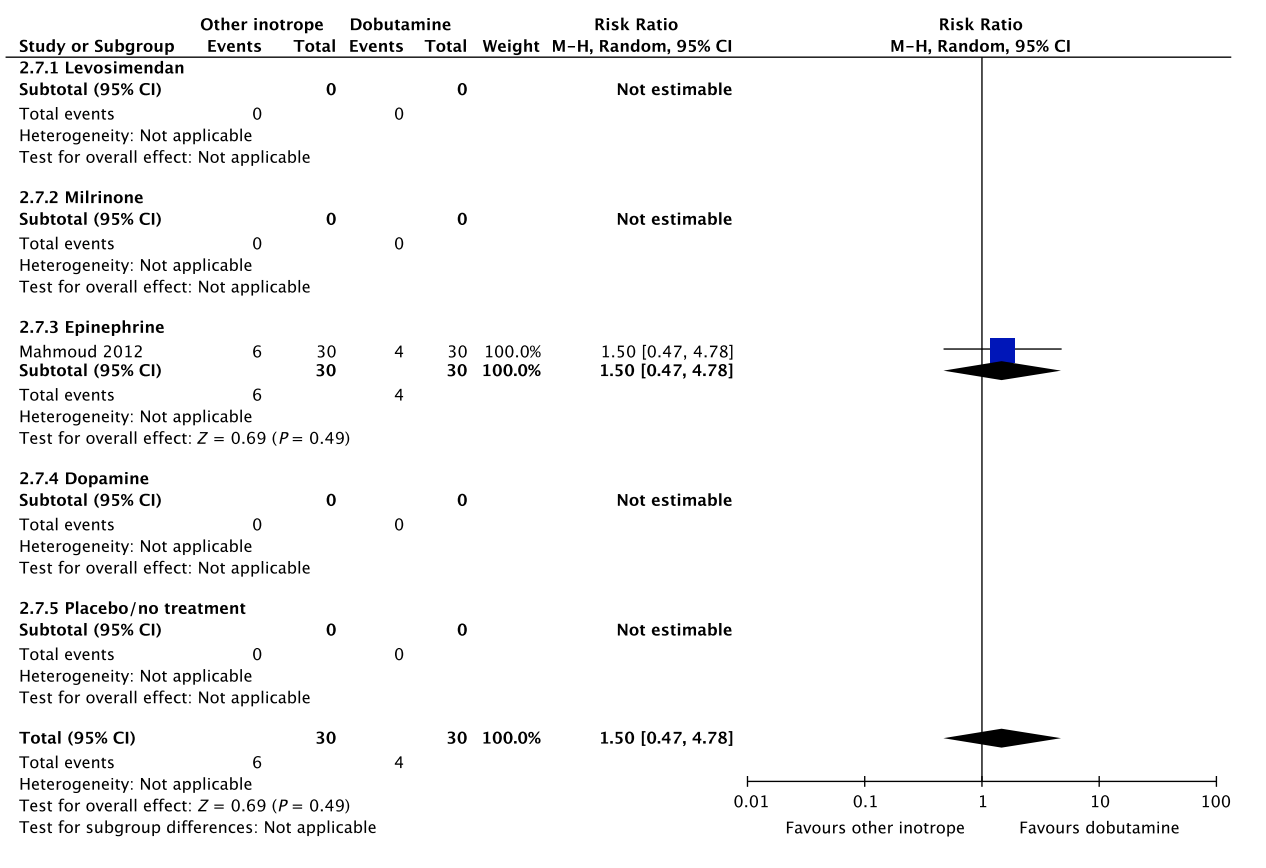
A small RCT comprising 60 patients with septic shock found no difference in short-term mortality, ischemic events, and dysrhythmias between patients treated with dobutamine vs. epinephrine (Fig. 2, Table S2C in Appendix S2).26 None of our other predefined patient-important outcome measures have been assessed. As excessive vasoconstriction and tachycardia may affect cardiac output adversely in most patients where an inotropic agent is deemed indicated,6 we suggest using dobutamine rather than epinephrine in patients with septic shock. The quality of evidence was downgraded due to imprecision, risk of bias and indirectness.

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with dopamine in patients with septic shock (Fig. 2, Table S2D in Appendix S2). We refrain from giving any recommendations or suggestions on using dobutamine or dopamine for patients with septic shock, due to the lack of data and no relevant populations to extrapolate from. Importantly, we recommend that if clinicians prefer to use dopamine rather than dobutamine in this population, they do so in the context of high-quality RCTs, given the harm associated with use of dopamine in patients with septic shock.17, 18

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with placebo/no treatment in patients with septic shock (Fig. 2, Table S2E in Appendix S2).
Importantly, potential harm of dobutamine has been suggested in a propensity-matched observational study in patients with septic shock.19 Consequently, we suggest against routine use of dobutamine as inotropic agent for patients with septic shock, as compared to placebo/no treatment.
The quality of evidence was downgraded due to serious risk of bias and indirectness.
A Short-term mortality
B Long-term mortality
No data.
C Quality of life
No data.
Fig. 2. Forest plot of (A) short-term mortality, (B) long-term mortality, (C) quality of life, (D) ischemic events, (E) renal replacement therapy, (F) acute kidney injury, (G) dysrhythmias, and (H) hospital length-of-stay in randomized trials of dobutamine vs. other inotropes for patients with septic shock. [Colour figure can be viewed at wileyonlinelibrary.com]
D Ischemic events
E Renal replacement therapy
No data.
F Acute kidney injury
No data.
Fig. 2. Continued
G Dysrhythmias
 H Hospital length-of-stay
H Hospital length-of-stay
No data.
Fig. 2. Continued
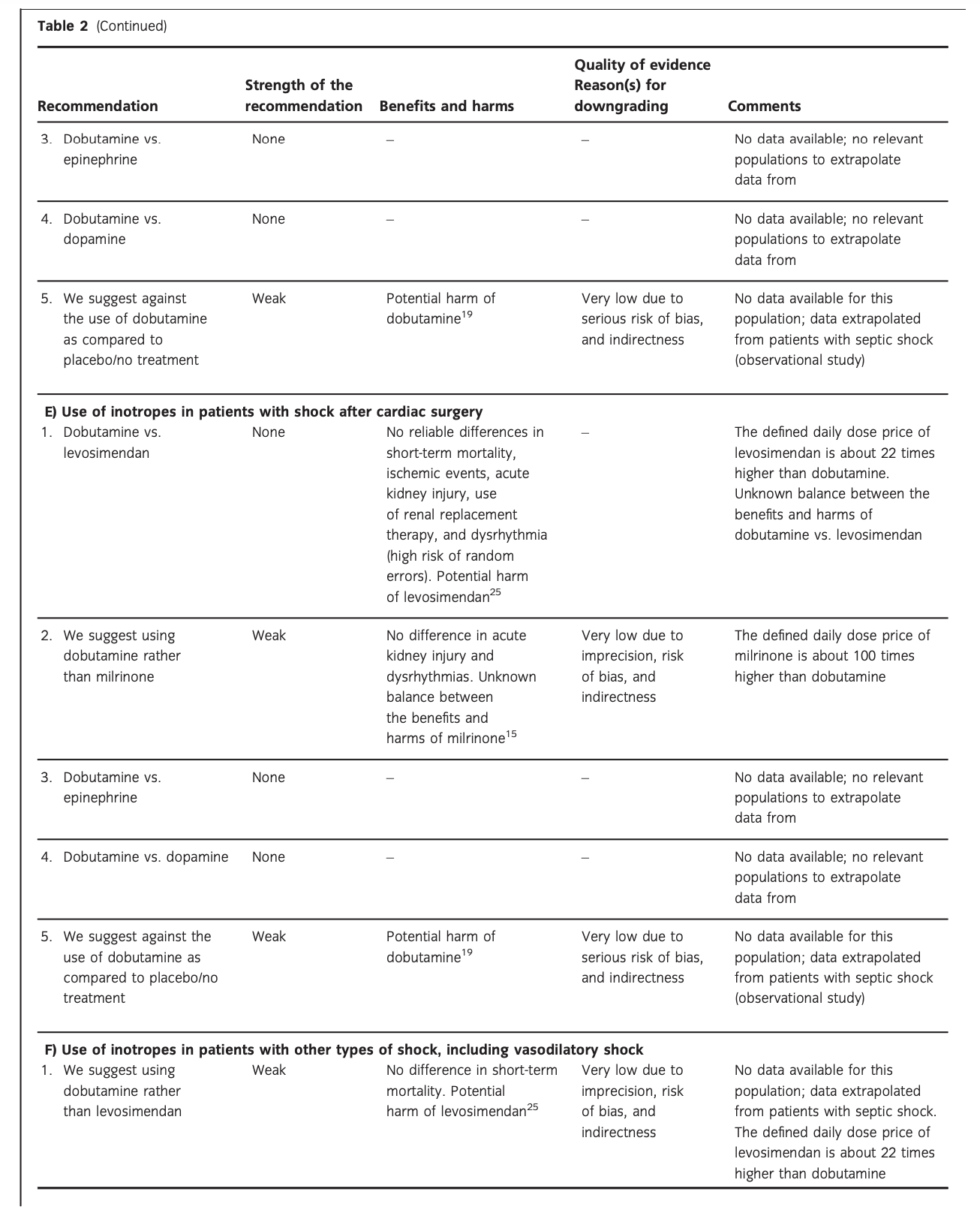
C. Dobutamine vs. other inotropes in patients with cardiogenic shock
In an updated meta-analysis comprising six trials, we found no statistically significant difference in short-term mortality,27–32 long-term mortality,27, 30, 31, 33–35 ischemic events,30, 34 acute kidney injury,31 dysrhythmias,30, 36 or hospital length-of-stay37 in patients with cardiogenic shock treated with dobutamine vs. levosimendan (Fig. 3, Fig. S2A, B, D, E in Appendix S1, Table S3A in Appendix S2). None of our other predefined patient-important outcome measures have been assessed. In the recently published LEOPARDS trial in which adult patients with sepsis were randomized to levosimendan or placebo, levosimendan was associated with a lower likelihood of successful weaning from mechanical ventilation and a higher risk of supraventricular tachyarrhythmia compared to placebo.25 Of note, the defined daily dose price of levosimendan is about 22 times higher than dobutamine.16 We recommend that if clinicians prefer to use levosimendan rather than dobutamine in this population, they do so in the context of high-quality RCTs, given the lack of data on the balance between the benefits and harms of levosimendan in patients with acute circulatory failure in general, the suggested harm of levosimendan in patients with sepsis,25 and the higher price.

A small RCT comprising 30 patients with cardiogenic shock38 found no difference in short-term mortality between patients treated with dobutamine vs. milrinone (Fig. 3, Table S3B in Appendix S2). None of our other predefined patient-important outcome measures have been assessed. As the balance between the benefits and harms of milrinone in patients with acute circulatory failure in general has been sparsely evaluated, we suggest using dobutamine rather than milrinone.15 The defined daily dose price of milrinone is about 100 times higher than that of dobutamine.16
The quality of evidence was downgraded due to imprecision, risk of bias, and indirectness

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with that of epinephrine in patients with cardiogenic shock (Fig. 3, Table S3C in Appendix S2).26 We refrain from giving any recommendations or suggestions on using dobutamine or epinephrine for patients with cardiogenic shock, due to the lack of data and no relevant populations to extrapolate from. Importantly, excessive vasoconstriction and tachycardia increase oxygen consumption and may affect cardiac output adversely in most patients where an inotropic agent is deemed indicated.6

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with dopamine in patients with cardiogenic shock (Fig. 3, Table S3D in Appendix S2). We refrain from giving any recommendations or suggestions on using dobutamine or dopamine for patients with cardiogenic shock, due to the lack of data and no relevant populations to extrapolate from. Importantly, we strongly recommend that if clinicians prefer to use dopamine rather than dobutamine in this population, they do so in the context of high-quality RCTs, given the harm associated with use of dopamine in patients with septic shock17,18 and in a subgroup analysis of patients with cardiogenic shock in the SOAP 2 trial.39

In an updated meta-analysis, we found no statistically significant difference in short-term mortality (1 trial, 199 patients)27 or long-term mortality (2 trials, 245 patients)27,35 in patients with cardiogenic shock treated with dobutamine vs. placebo/no treatment (Fig. 3, Fig. S2C in Appendix S1, Table S3E in Appendix S2). TSA highlighted high risk of random errors due to repetitive testing and small sample sizes (Fig. S2 in Appendix S1), which cautions interpretations of the findings in the conventional meta-analysis. None of our other predefined patient-important outcome measures have been assessed. Importantly, as potential harm of dobutamine has been suggested in patients with septic shock, we suggest against the routine use of dobutamine as inotropic agent for patients with cardiogenic shock, as compared to placebo/no treatment (extrapolation).
The quality of evidence was downgraded due to imprecision, indirectness, and risk of bias.
D. Dobutamine vs. other inotropes in patients with hypovolemic shock

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with levosimendan in patients with hypovolemic shock (Fig. 4, Table S4A in Appendix S2). We refrain from giving any recommendations or suggestions on using dobutamine or levosimendan for patients with hypovolemic shock, due to the lack of data and no relevant populations to extrapolate from. In the recently published LEOPARDS trial in which adult patients with sepsis were randomized to levosimendan or placebo, levosimendan was associated with a lower likelihood of successful weaning from mechanical ventilation and a higher risk of supraventricular tachyarrhythmia compared to placebo.25 This cautions use of levosimendan in other patient groups, including patients with hypovolemic shock. Of note, the defined daily dose price of levosimendan is about 22 times higher than dobutamine.16 Importantly, adequate fluid resuscitation – and not inodilation – should be a priority in patients with hypovolemic shock.

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with milrinone in patients with hypovolemic shock (Fig. 4, Table S4B in Appendix S2). We refrain from giving any recommendations or suggestions on using dobutamine or milrinone for patients with hypovolemic shock, due to the lack of data and no relevant populations to extrapolate from. We recommend that if clinicians prefer to use milrinone rather than dobutamine in this population, they do so in the context of high-quality RCTs, given the lack of data on the balance between benefits and harms of milrinone in patients with acute circulatory failure in general.15 Of note, the defined daily dose price of milrinone is about 100 times higher than that of dobutamine.16 Importantly, adequate fluid resuscitation – and not inodilation – should be a priority in patients with hypovolemic shock.

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with that of epinephrine in patients with cardiogenic shock (Fig. 3, Table S3C in Appendix S2).26 We refrain from giving any recommendations or suggestions on using dobutamine or epinephrine for patients with cardiogenic shock, due to the lack of data and no relevant populations to extrapolate from. Importantly, excessive vasoconstriction and tachycardia increase oxygen consumption and may affect cardiac output adversely in most patients where an inotropic agent is deemed indicated.6

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with dopamine in patients with cardiogenic shock (Fig. 3, Table S3D in Appendix S2). We refrain from giving any recommendations or suggestions on using dobutamine or dopamine for patients with cardiogenic shock, due to the lack of data and no relevant populations to extrapolate from. Importantly, we strongly recommend that if clinicians prefer to use dopamine rather than dobutamine in this population, they do so in the context of high-quality RCTs, given the harm associated with use of dopamine in patients with septic shock17,18 and in a subgroup analysis of patients with cardiogenic shock in the SOAP 2 trial.39

In an updated meta-analysis, we found no statistically significant difference in short-term mortality (1 trial, 199 patients)27 or long-term mortality (2 trials, 245 patients)27,35 in patients with cardiogenic shock treated with dobutamine vs. placebo/no treatment (Fig. 3, Fig. S2C in Appendix S1, Table S3E in Appendix S2). TSA highlighted high risk of random errors due to repetitive testing and small sample sizes (Fig. S2 in Appendix S1), which cautions interpretations of the findings in the conventional meta-analysis. None of our other predefined patient-important outcome measures have been assessed. Importantly, as potential harm of dobutamine has been suggested in patients with septic shock, we suggest against the routine use of dobutamine as inotropic agent for patients with cardiogenic shock, as compared to placebo/no treatment (extrapolation). The quality of evidence was downgraded due to imprecision, indirectness, and risk of bias.
A Short-term mortality
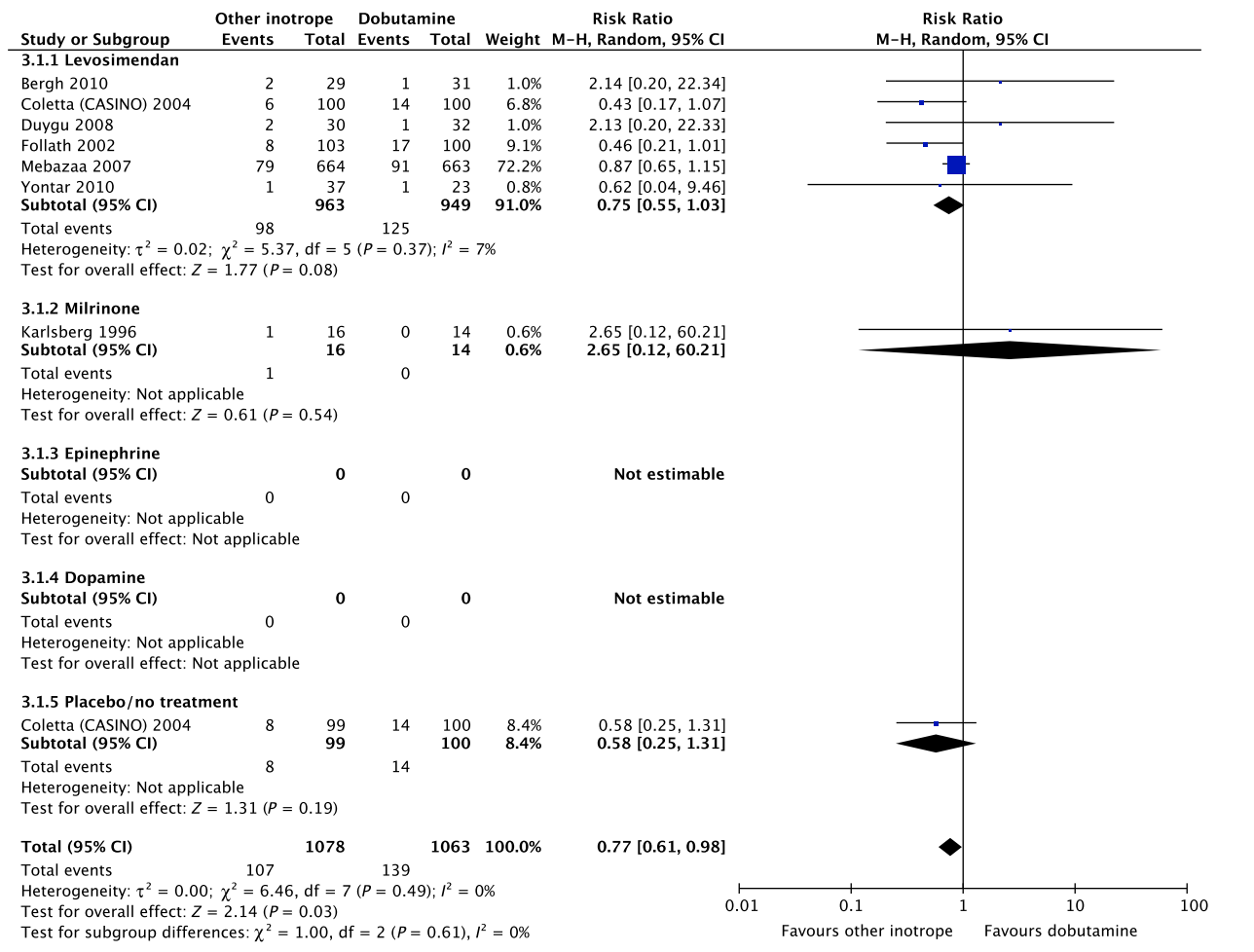
Fig. 3. Forest plot of (A) short-term mortality, (B) long-term mortality, (C) quality of life, (D) ischemic events, (E) renal replacement therapy, (F) acute kidney injury, (G) dysrhythmias, and (H) hospital length-of-stay in randomized trials of dobutamine vs. other inotropes for patients with cardiogenic shock. [Colour figure can be viewed at wileyonlinelibrary.com]
B Long-term mortality
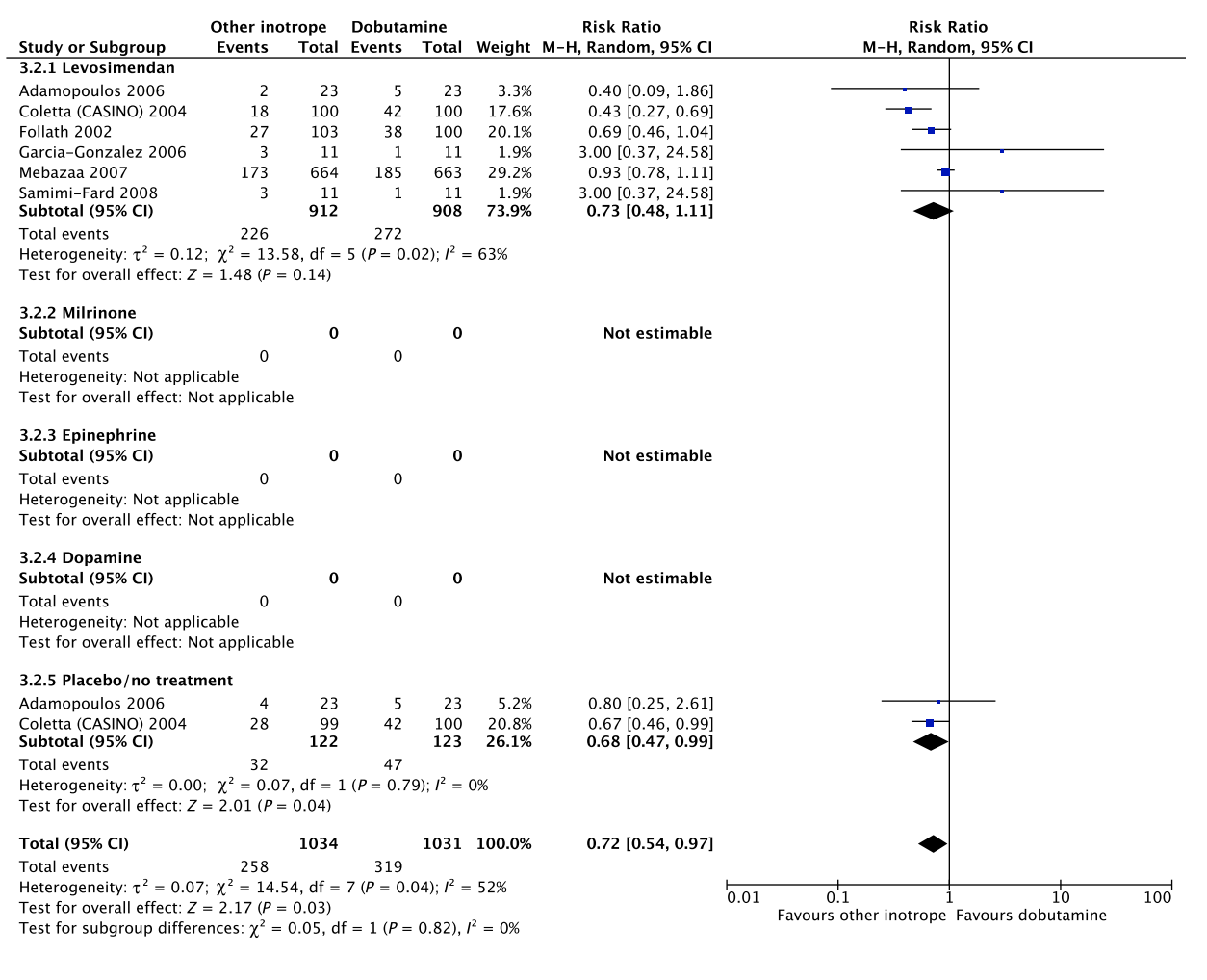
C Quality of life
No data.
Fig. 3. Continued
D Ischemic events
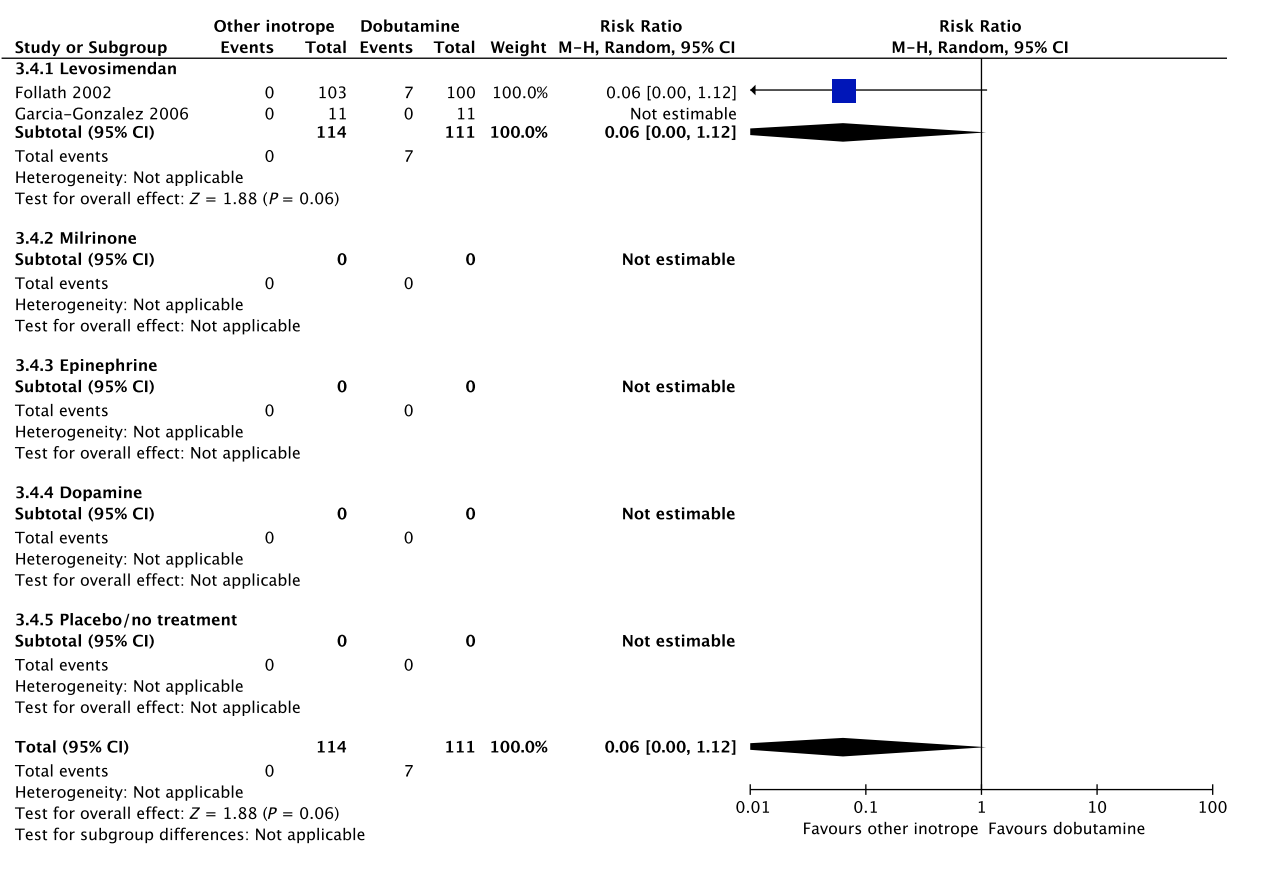
E Renal replacement therapy No data.
Fig. 3. Continued
F Acute kidney injury
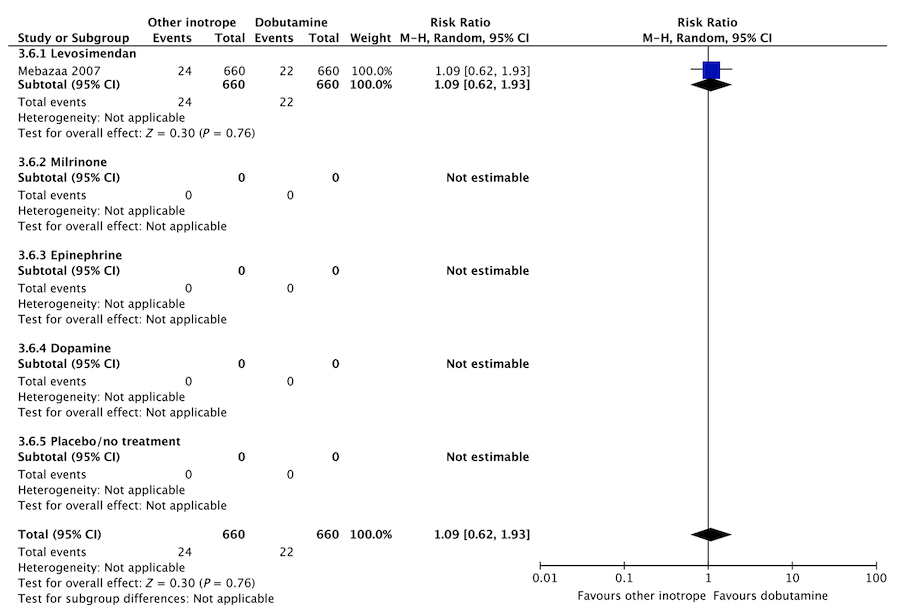
Fig. 3. Continued
G Dysrhythmias
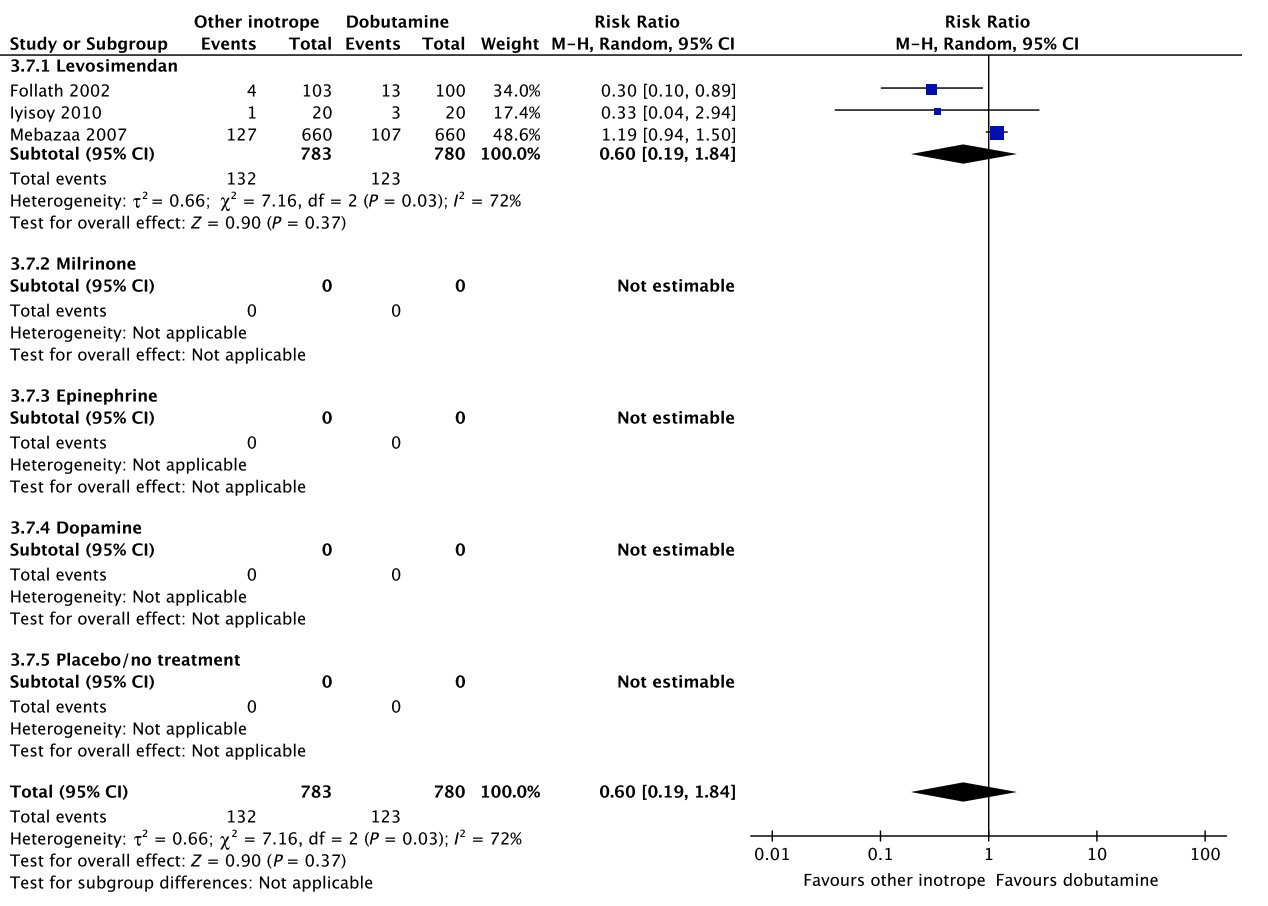
Fig. 3. Continued
H Hospital length-of-stay
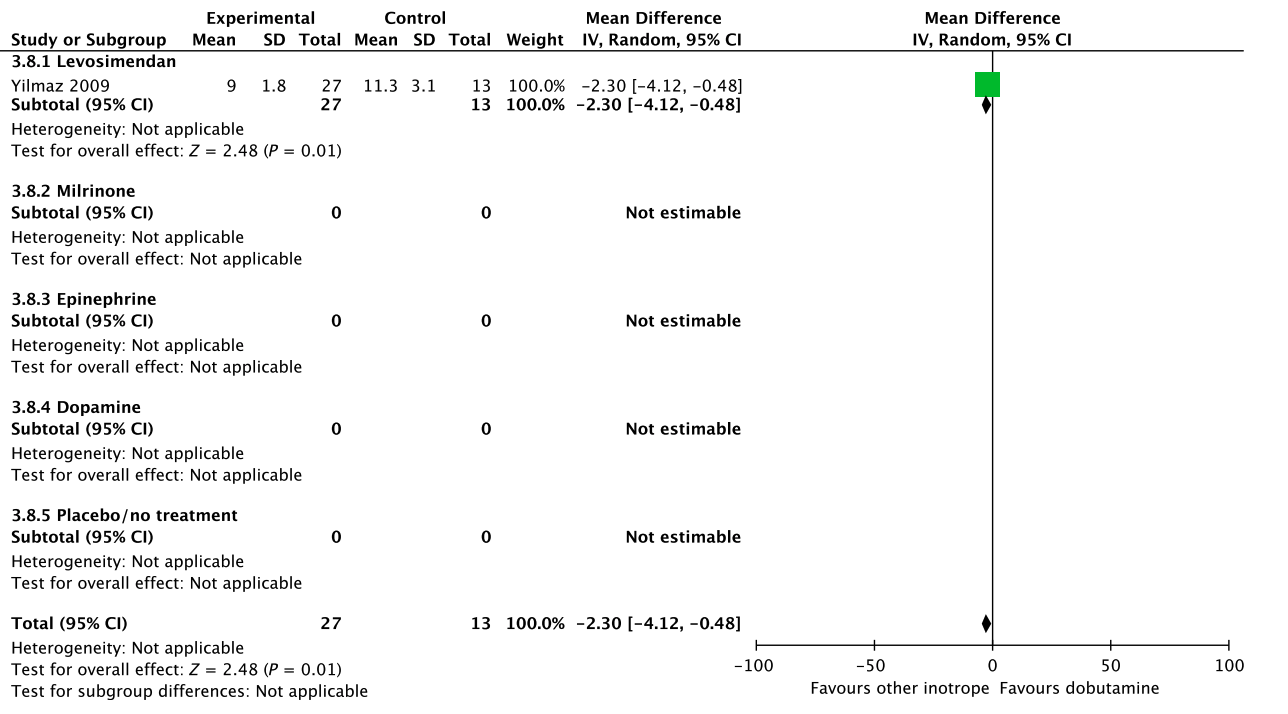
Fig. 3. Continued
D. Dobutamine vs. other inotropes in patients with hypovolemic shock

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with levosimendan in patients with hypovolemic shock (Fig. 4, Table S4A in Appendix S2). We refrain from giving any recommendations or suggestions on using dobutamine or levosimendan for patients with hypovolemic shock, due to the lack of data and no relevant populations to extrapolate from. In the recently published LEOPARDS trial in which adult patients with sepsis were randomized to levosimendan or placebo, levosimendan was associated with a lower likelihood of successful weaning from mechanical ventilation and a higher risk of supraventricular tachyarrhythmia compared to placebo.25 This cautions use of levosimendan in other patient groups, including patients with hypovolemic shock. Of note, the defined daily dose price of levosimendan is about 22 times higher than dobutamine.16 Importantly, adequate fluid resuscitation – and not inodilation – should be a priority in patients with hypovolemic shock.

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with milrinone in patients with hypovolemic shock (Fig. 4, Table S4B in Appendix S2). We refrain from giving any recommendations or suggestions on using dobutamine or milrinone for patients with hypovolemic shock, due to the lack of data and no relevant populations to extrapolate from. We recommend that if clinicians prefer to use milrinone rather than dobutamine in this population, they do so in the context of high-quality RCTs, given the lack of data on the balance between benefits and harms of milrinone in patients with acute circulatory failure in general.15 Of note, the defined daily dose price of milrinone is about 100 times higher than that of dobutamine.16 Importantly, adequate fluid resuscitation – and not inodilation – should be a priority in patients with hypovolemic shock.

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with epinephrine in patients with hypovolemic shock (Fig. 4, Table S4C in Appendix S2).26 We refrain from giving any recommendations or suggestions on using dobutamine or epinephrine for patients with hypovolemic shock, due to the lack of data and no relevant populations to extrapolate from. Importantly, excessive vasoconstriction and tachycardia increase oxygen consumption and may affect cardiac output adversely in most patients where an inotropic agent is deemed indicated.6 Importantly, adequate fluid resuscitation should be a priority in patients with hypovolemic shock.

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with dopamine in patients with hypovolemic shock (Fig. 4, Table S4D in Appendix S2). We refrain from giving any recommendations or suggestions on using dobutamine or dopamine for patients with hypovolemic shock, due to the lack of data and no relevant populations to extrapolate from. Importantly, we strongly recommend that if clinicians prefer to use dopamine rather than dobutamine in this population, they do so in the context of high-quality RCTs, given the harm associated with use of dopamine in patients with septic shock17, 18 and in a subgroup analysis of patients with cardiogenic shock in the SOAP 2 trial.39 Importantly, adequate fluid resuscitation should be a priority in patients with hypovolemic shock.

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with placebo/no treatment in patients with hypovolemic shock (Fig. 4, Table S4E in Appendix S2). Importantly, potential harm of dobutamine has been suggested in a propensity-matched observational study in patients with septic shock.19 In reference to our recommendation for patients with septic shock, we suggest against routine use of dobutamine (extrapolation). Of note, adequate fluid resuscitation should be a priority in patients with hypovolemic shock. The quality of evidence was downgraded due to serious risk of bias and indirectness.
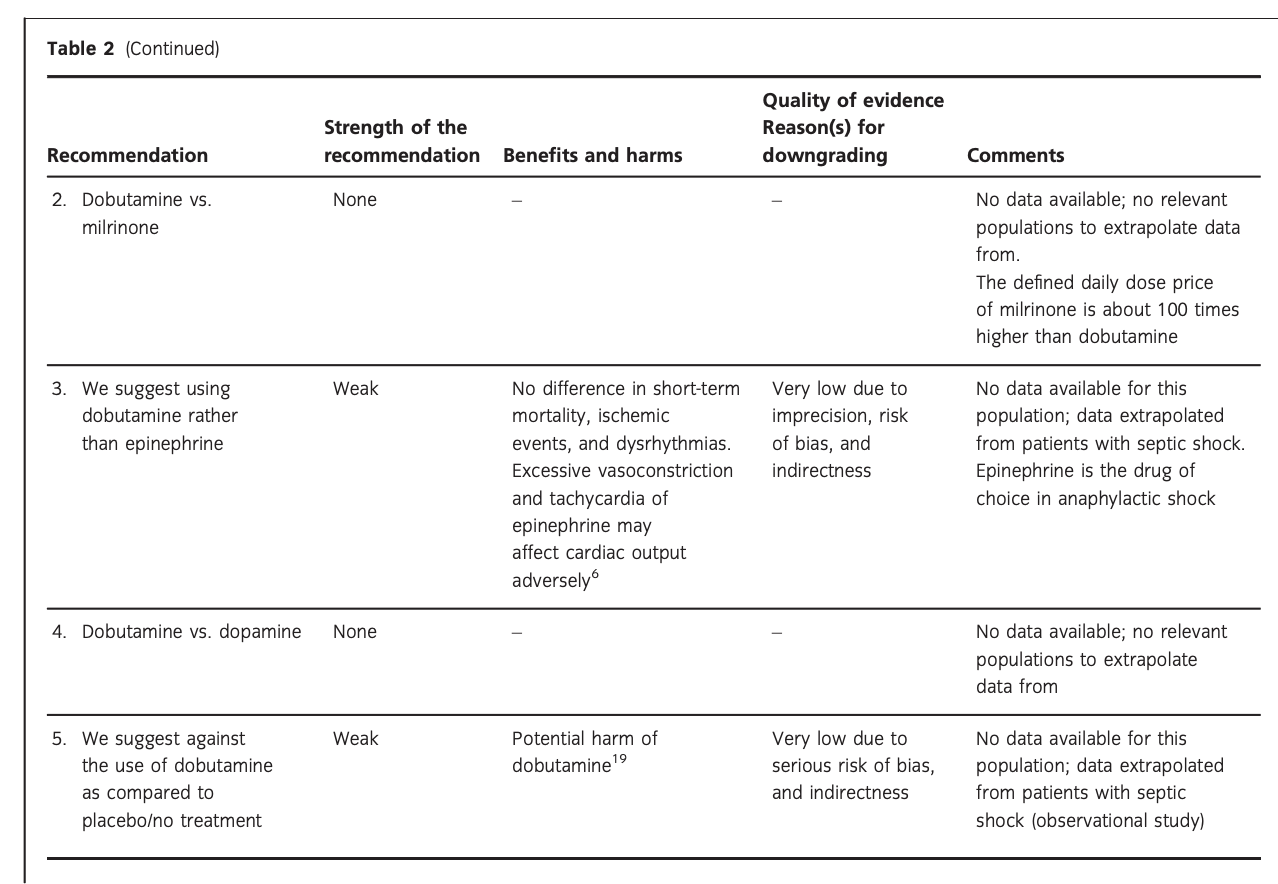
E. Dobutamine vs. other inotropes in patients with shock after cardiac surgery

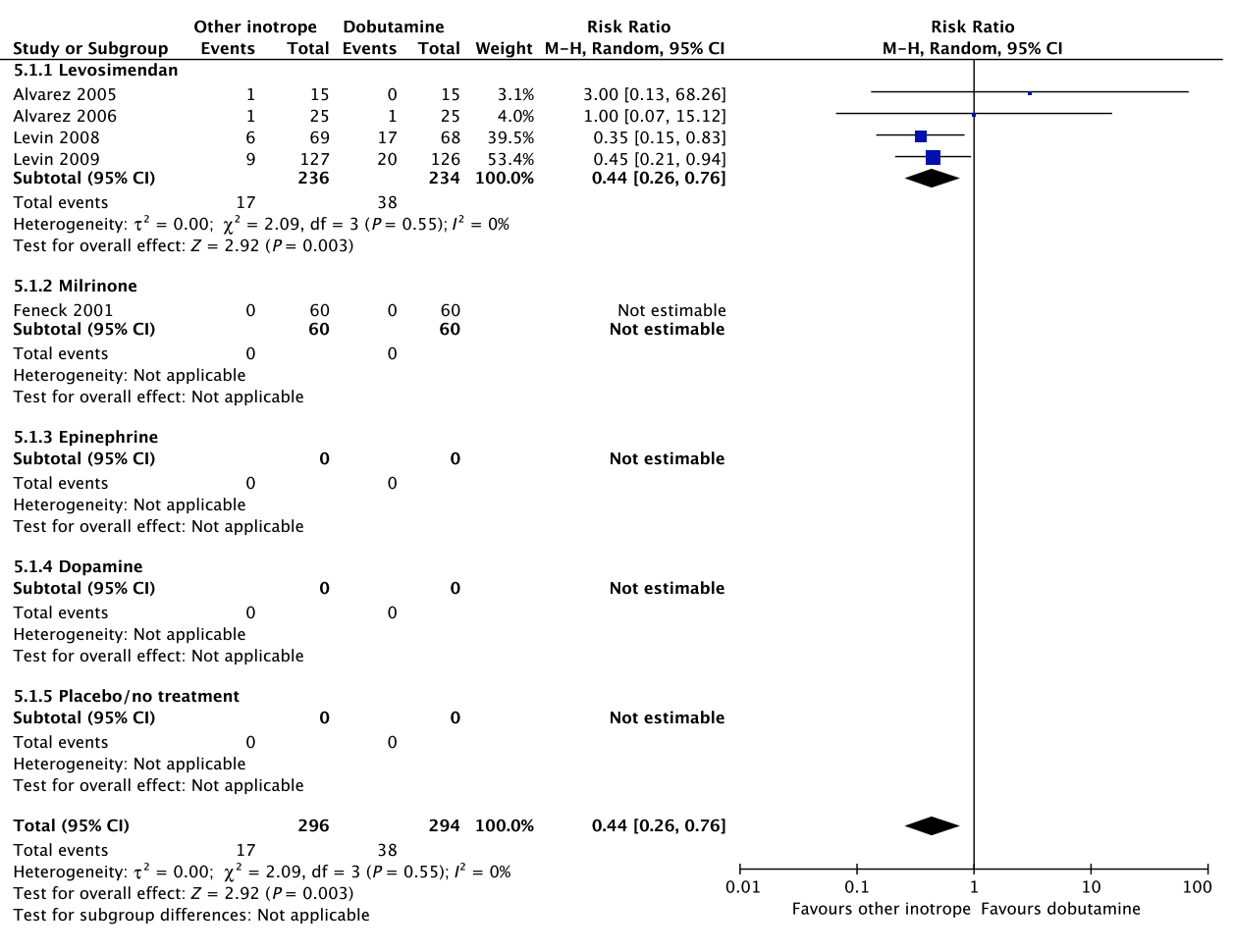
In an updated meta-analysis comprising four trials and 470 patients, reduced short-term mortality,40–43 fewer ischemic events,43 reduced risk of acute kidney injury and use of renal replacement therapy,40–43 and reduced risk of dysrhythmias40–43 were suggested in patients with shock after cardiac surgery treated with levosimendan, as compared to dobutamine (Fig. 5, Fig. S3A–C in Appendix S1, Table S5A in Appendix S2). However, TSA highlighted high risk of random errors due to repetitive testing and small sample sizes (Fig. S3 in Appendix S1), which cautions interpretations of the findings in the conventional meta-analysis. None of our other predefined patient-important outcome measures have been assessed. In the recently published LEVO-CTS, CHEETAH, and LICORN trials44–46 no difference in outcome between levosimendan and placebo in patients undergoing planned cardiac surgery was found. Of note, levosimendan was studied as a second-line inotropic agent in these trials, and other inotropic drugs, such as dobutamine, were permitted. Importantly, in the LEOPARDS trial in which adult patients with sepsis where randomized to levosimendan or placebo, levosimendan was associated with a lower likelihood of successful weaning from mechanical ventilation and a higher risk of supraventricular tachyarrhythmia, as compared to placebo.25 Of note, the defined daily dose price of levosimendan is about 22 times higher than dobutamine.16 We refrain from giving any recommendations or suggestions on using dobutamine or levosimendan for patients with shock after cardiac surgery, due to the unknown balance between the benefits and harms of these agents in this population.

A small RCT comprising 120 patients with shock after cardiac surgery47 found no difference in acute kidney injury and dysrhythmias between patients treated with dobutamine vs. milrinone (Fig. 5, Table S5B in Appendix S2). None of our other predefined patient-important outcome measures have been assessed. As the balance between the benefits and harms of milrinone in patients with acute circulatory failure in general has been sparsely evaluated,15 we suggest using dobutamine rather than milrinone. Furthermore, the defined daily dose price of milrinone is about 100 times higher than that of dobutamine.16 The quality of evidence was downgraded due to imprecision, indirectness, and risk of bias.
No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with epinephrine in patients with shock post–cardiac surgery (Fig. 5, Table S5C in Appendix S2).26 We refrain from giving any recommendations or suggestions on using dobutamine or epinephrine for patients with shock after cardiac surgery, due to the lack of data and no relevant populations to extrapolate from. Importantly, excessive vasoconstriction and tachycardia increase oxygen consumption and may affect cardiac output adversely in most patients where an inotropic agent is deemed indicated.6

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with dopamine in patients with shock after cardiac surgery (Fig. 5, Table S5D in Appendix S2). We refrain from giving any recommendations or suggestions on using dobutamine or dopamine for patients with shock after cardiac surgery, due to the lack of data and no relevant populations to extrapolate from. Importantly, we recommend that if clinicians prefer to use dopamine rather than dobutamine in this population, they do so in the context of high-quality RCTs, given the harm associated with use of dopamine in patients with septic shock17, 18 and in a subgroup analysis of patients with cardiogenic shock in the SOAP 2 trial.39

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with placebo/no treatment in patients with shock after cardiac surgery (Fig. 5, Table S5E in Appendix S2). Importantly, as potential harm of dobutamine has been suggested in patients with septic shock, we suggest against the routine use of dobutamine as inotropic agent for patients with shock after cardiac surgery, as compared to placebo/no treatment (extrapolation). The quality of evidence was downgraded due to serious risk of bias and indirectness.
A Short-term mortality
No data.
B Long-term mortality
No data.
C Quality of life
No data.
D Ischemic events
No data.
E Renal replacement therapy
No data.
F Acute kidney injury
No data.
G Dysrhythmias
No data.
H Hospital length-of-stay
No data.
Fig. 4. Forest plot of (A) short-term mortality, (B) long-term mortality, (C) quality of life, (D) ischemic events, (E) renal replacement therapy, (F) acute kidney injury, (G) dysrhythmias, and (H) hospital length-ofstay in randomized trials of dobutamine vs. other inotropes for patients with hypovolemic shock.
A Short-term mortality
B Long-term mortality
No data.
C Quality of life
No data.
Fig. 5. Forest plot of (A) short-term mortality, (B) long-term mortality, (C) quality of life, (D) ischemic events, (E) renal replacement therapy, (F) acute kidney injury, (G) dysrhythmias, and (H) hospital length-of-stay in randomized trials of dobutamine vs. other inotropes for patients with shock after cardiac surgery. [Colour figure can be viewed at wileyonlinelibrary.com]
D Ischemic events
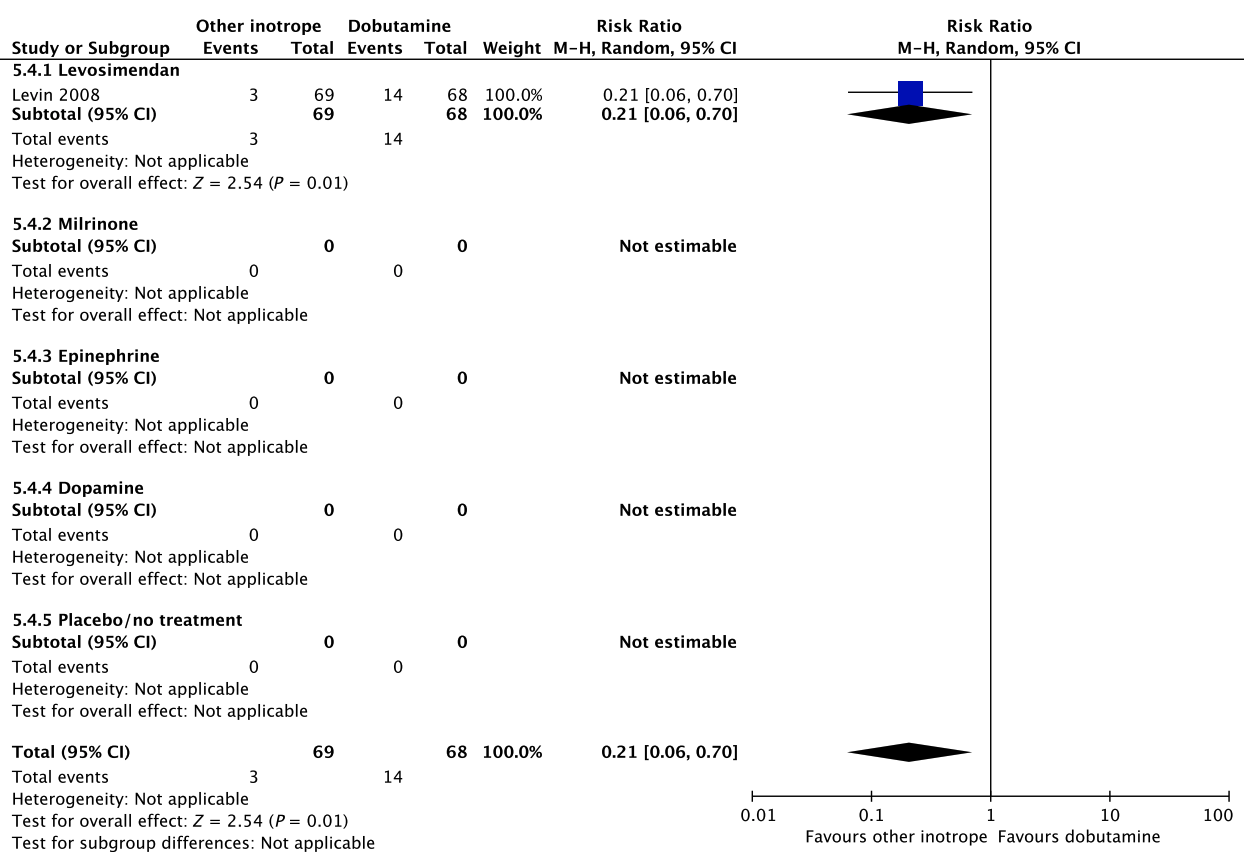
Fig. 5. Continued
E Renal replacement therapy
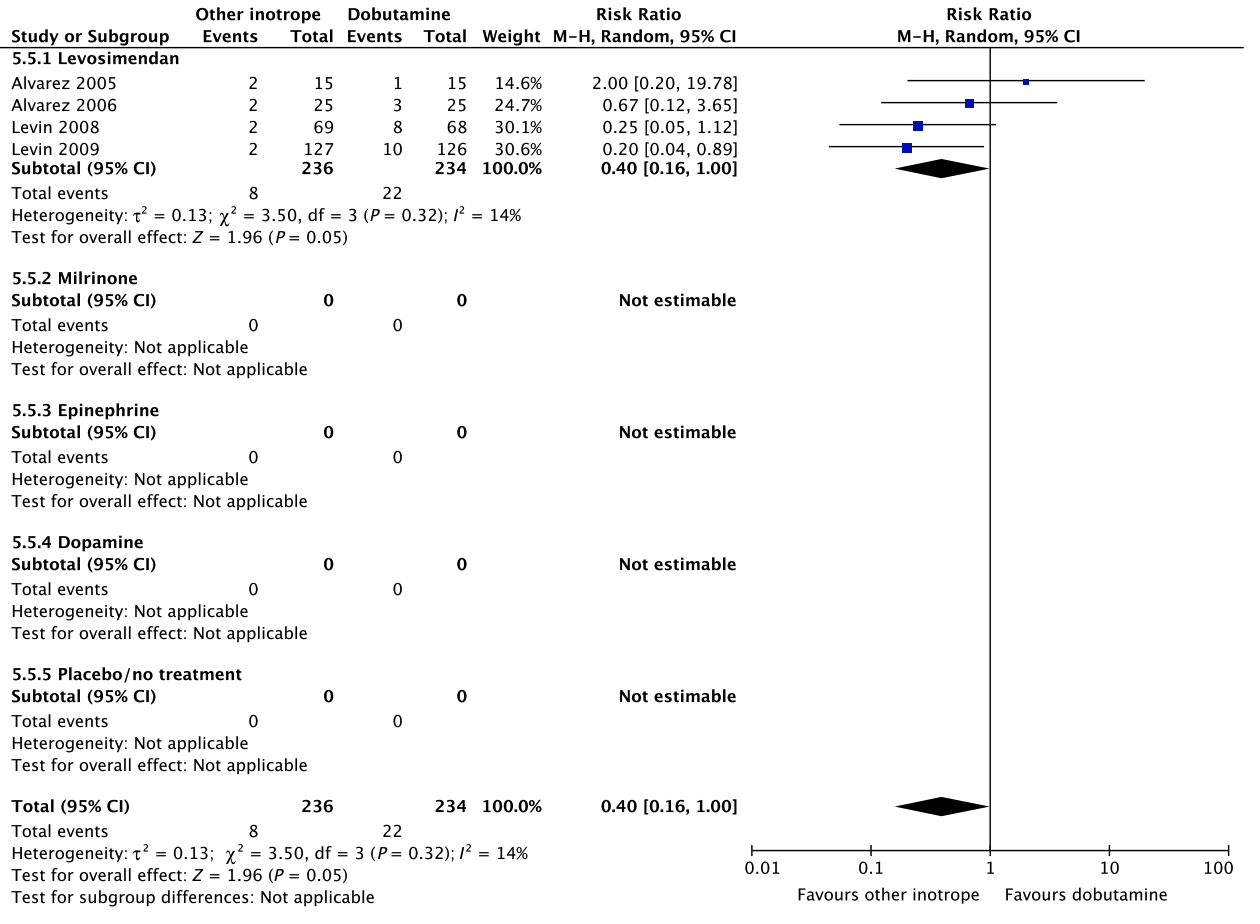
Fig. 5. Continued
F. Dobutamine vs. other inotropes in patients with other types of shock, including vasodilatory shock

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with levosimendan in patients with other types of shock including vasodilatory shock (Fig. 6, Table S6A in Appendix S2). In reference to our recommendation for patients with septic shock, we suggest using dobutamine (extrapolation). The quality of evidence was downgraded due to risk of bias, indirectness, and imprecision.

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with milrinone in patients with other types of shock including vasodilatory shock (Fig. 6, Table S6B in Appendix S2). We refrain from giving any recommendations or suggestions on using dobutamine or milrinone for patients with other types of shock including vasodilatory shock, due to the lack of data and no relevant populations to extrapolate from. Importantly, we recommend that if clinicians prefer to use milrinone rather than dobutamine in this population, they do so in the context of high-quality RCTs, given the lack of data on the balance between benefits and harms of milrinone in patients with acute circulatory failure in general.15 Of note, the defined daily dose price of milrinone is about 100 times higher than that of dobutamine.16

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with epinephrine in patients with other types of shock including vasodilatory shock (Fig. 6, Table S6C in Appendix S2). In reference to our recommendation for patients with septic shock, we suggest using dobutamine (extrapolation). Importantly, in vasodilatory shock caused by anaphylaxis, epinephrine is the preferred drug of choice. The quality of evidence was downgraded due to risk of bias, indirectness, and imprecision.

No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with dopamine in patients with other types of shock including vasodilatory shock (Fig. 6, Table S6D in Appendix S2). We refrain from giving any recommendations or suggestions on using dobutamine or dopamine for patients with other types of shock including vasodilatory shock, due to the lack of data and no relevant populations to extrapolate from. Importantly, we strongly recommend that if clinicians prefer to use dopamine rather than dobutamine in this population, they do so in the context of high-quality RCTs, given the harm associated with use of dopamine in patients with septic shock17, 18 and in a subgroup analysis of patients with cardiogenic shock in the SOAP 2 trial.39
No systematic reviews or RCTs reporting our predefined patient-important outcome measures have compared use of dobutamine with placebo in patients with other types of shock including vasodilatory shock (Fig. 6, Table S6E in Appendix S2). In reference to our recommendation for patients with septic shock, we suggest against routine use of dobutamine (extrapolation). The quality of evidence was downgraded due to serious risk of bias and indirectness.
F Acute kidney injury
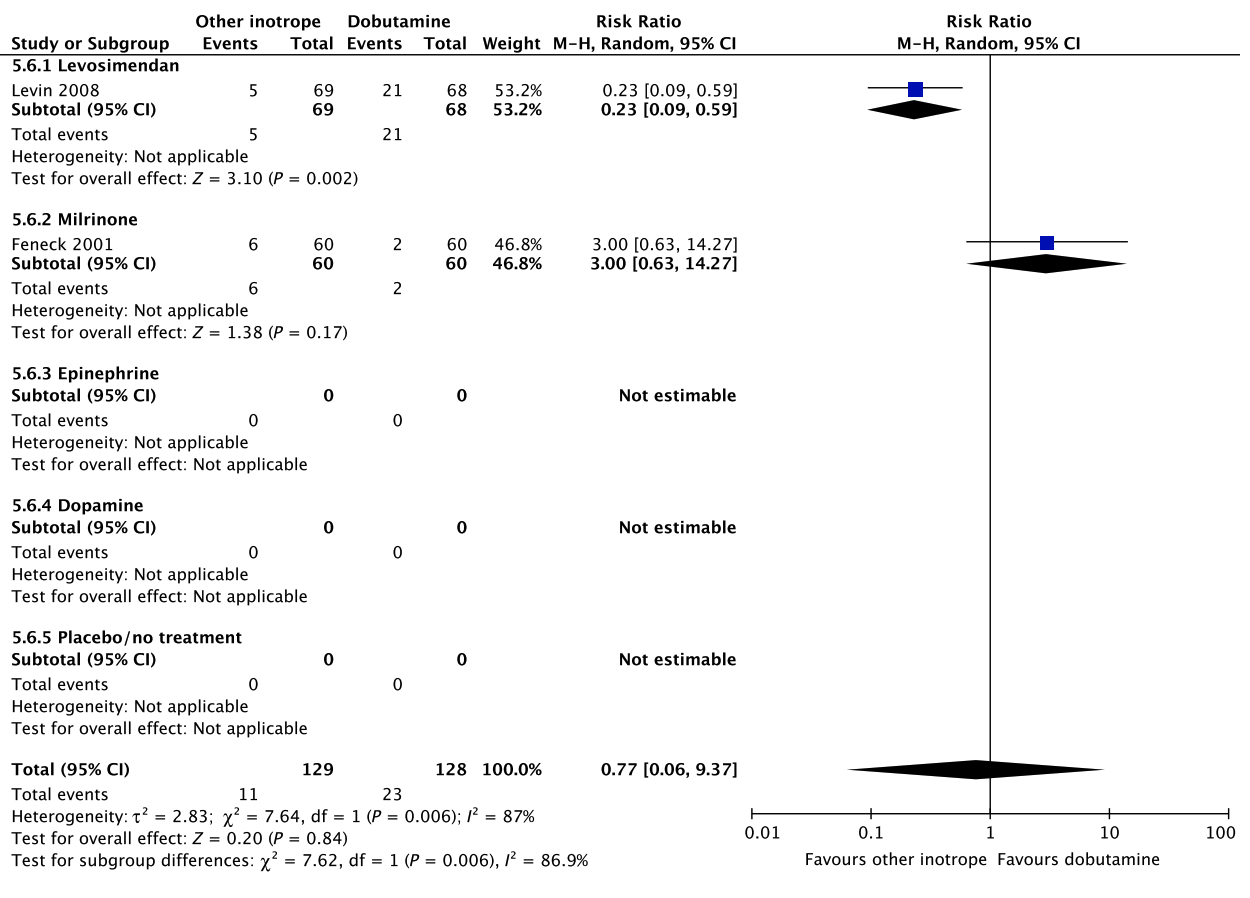
Fig. 5. Continued
G Dysrhythmias
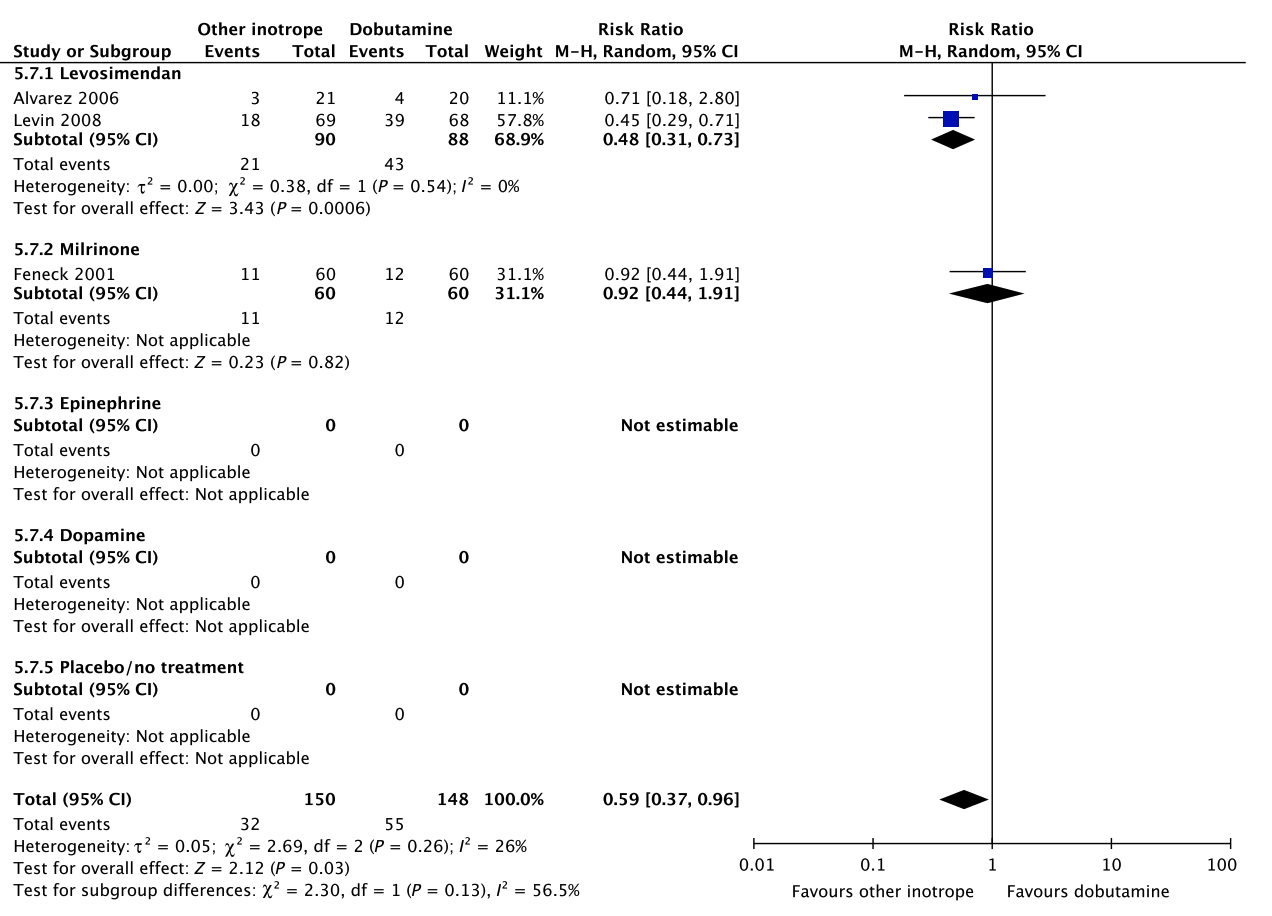
H Hospital length-of-stay
No data.
Fig. 5. Continued
A Short-term mortality
No data.
B Long-term mortality
No data.
C Quality of life
No data.
D Ischemic events
No data.
E Renal replacement therapy
No data.
F Acute kidney injury
No data.
G Dysrhythmias
No data.
H Hospital length-of-stay
No data.
Fig. 6. Forest plot of (A) short-term mortality, (B) long-term mortality, (C) quality of life, (D) ischemic events, (E) renal replacement therapy, (F) acute kidney injury, (G) dysrhythmias, and (H) hospital length-ofstay in randomized trials of dobutamine vs. other inotropes for patients with other types of shock, including vasodilatory.
Discussion
We were able to use existing systematic reviews and RCTs to answer some of the clinical questions concerning choice of inotropic agents in patients with septic shock, cardiogenic shock, and in those with shock after cardiac surgery. However, for patients with shock in general, and those with hypovolemic shock, and other types of shock, the quantity and quality of evidence was very limited.
The most widely studied comparison was dobutamine vs. levosimendan, whereas dobutamine vs. milrinone, dobutamine vs. epinephrine, and dobutamine vs. placebo/no treatment have been sparsely assessed. No trials have compared dobutamine vs. dopamine in any of the six predefined subpopulations.
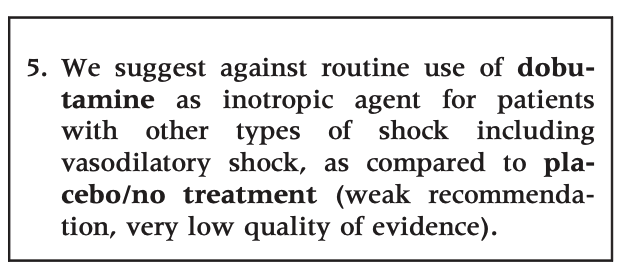
We propose no strong recommendations, as the quantity and quality of evidence was very low with large uncertainty about the direction and magnitude of effect. For all the six predefined subpopulations, we suggest against the routine use of dobutamine, as compared to placebo/no treatment (very low quality of evidence). There are no data supporting that inotropic agents offer benefit as compared to placebo or no treatment, and in patients with septic shock, dobutamine has been associated with adverse outcome.
For patients with shock in general and those with septic shock and other types of shock, we suggest using dobutamine over levosimendan (very low quality of evidence). This was based on an overall low confidence of benefit from levosimendan, and importantly, potential harm, as suggested in the LEOPARDS trial, in which adult patients with sepsis randomized to treatment with levosimendan had lower likelihood of successful weaning from mechanical ventilation and a higher rate of supraventricular tachyarrhythmia, as compared to placebo.25
For patients with shock in general and in those with septic and other types of shock, we suggest using dobutamine over epinephrine, as excessive vasoconstriction and tachycardia may affect oxygen consumption and cardiac output adversely in most patients where an inotropic agent is deemed indicated (very low quality of evidence).
For patients with cardiogenic shock and those with shock after cardiac surgery, we suggest using dobutamine over milrinone (very low quality of evidence). This was based on overall low confidence of benefit and insufficient knowledge on harms from milrinone,15 and a considerably higher defined daily dose price of milrinone.
Because of no available data, no reliable data on the balance between the benefits and harms, or no relevant patient groups to extrapolate from, we were not able to provide recommendations/suggestions for (1) dobutamine vs. milrinone/dopamine in patients with shock in general, and for those with septic and other types of shock, (2) dobutamine vs. levosimendan/epinephrine/dopamine for patients with cardiogenic shock and those with shock after cardiac surgery, and for (3) dobutamine vs. levosimendan/milrinone/epinephrine/dopamine in patients with hypovolemic shock.
As witnessed by the very low quality of evidence supporting the suggestions of this guideline, there is large uncertainty on the balance between the benefits and harms when using inotropic agents in adult patients with acute circulatory failure.48 Several interventions, which are common practice in the ICU, have been adopted based on the perception of improved physiological parameters and physiological reasoning. This has the eminent risk of overestimating benefit and underestimating harm.49 In a recently published systematic review, eight critical care interventions used in clinical practice were shown to increase mortality.50 Furthermore, there is empirical evidence within critical care that research results based on data from trials with lower quality have changed direction once higher quality trials were published.51 Consequently, we highly recommend that clinicians who are using inotropic agents in patients with acute circulatory failure, consider doing this in the context of high-quality RCTs with low risk of bias-assessing patient-important outcomes. The strengths of this clinical practice guideline include the application of current standards for trustworthy guidelines, including the GRADE methodology which support a systematic and transparent process,9 and use of TSA to assess the risk of random errors.13 The limitations include the reliance upon existing systematic reviews for some recommendations, including the risk of trial heterogeneity, indirectness, and bias. Also, we did not include time to resolution of shock or days free of inotropic support as outcomes, as we did not expect that any trials had assessed these otherwise patient-important outcomes. Furthermore, not all of the included systematic reviews and trials have been designed as a direct comparison between dobutamine and another inotropic agent, as some trials have used adjuvant vasopressors. Consequently, some of the benefits and harms observed may partly be caused by other adjuvant agents used and/or induced changes in dosing of the inotropic agent assessed. Our recommendations have been restricted to those that can be based on findings from RCTs exclusively, however, observational studies may – although seldom and often biased – provide evidence to help form some recommendations.52 Finally, our guideline group did not include critical care nurses or other relevant stakeholders such as patients, relatives, and representatives of regulatory bodies and hospital owners.
Conclusion
For all adult patients with shock, we suggest against the routine use of dobutamine as compared to placebo/no treatment. If inotropic agents are used, we suggest using dobutamine rather than levosimendan or epinephrine in patients with shock in general, and in those with septic and other types of shock. In patients with cardiogenic shock and in those with shock after cardiac surgery, we suggest using dobutamine rather than milrinone. For the remaining clinical questions, we refrained from giving any recommendations or suggestions. In general, the quality of evidence was very low, implying high uncertainty on the balance between the benefits and harms when using inotropes in adult patients with acute circulatory failure. Consequently, RCTs with low risk of bias should be a high research priority in settings where inotropes are used.
Acknowledgements
None.
References
- Vincent JL, De Backer D. Circulatory shock. N Engl J Med 2013; 369: 1726–34.
- Perner A, Junttila E, Haney M, Hreinsson K, Kvale R, Vandvik PO, Moller MH, Scandinavian Society of Anaesthesiology and Intensive Care Medicine. Scandinavian clinical practice guideline on choice of fluid in resuscitation of critically ill patients with acute circulatory failure. Acta Anaesthesiol Scand 2015; 59: 274–85.
- Moller MH, Claudius C, Junttila E, Haney M, Oscarsson-Tibblin A, Haavind A, Perner A. Scandinavian SSAI clinical practice guideline on choice of first-line vasopressor for patients with acute circulatory failure. Acta Anaesthesiol Scand 2016; 60: 1347–66.
- Rochwerg B, Hylands M, Moller M, Asfar P, Cohen D, Khadaroo RG, Laake JH, Perner A, Tanguay T, Widder S, Vandvik P, Kristiansen A, Lamontagne F. CCCS-SSAI WikiRecs Clinical Practice Guideline: vasopressor blood pressure targets in critically ill adults with hypotension. Can J Anaesth 2017; 64: 763–5.
- Arrigo M, Mebazaa A. Understanding the differences among inotropes. Intensive Care Med 2015; 41: 912–5.
- Jentzer JC, Coons JC, Link CB, Schmidhofer M. Pharmacotherapy update on the use of vasopressors and inotropes in the intensive care unit. J Cardiovasc Pharmacol Ther 2015; 20: 249–60.
- Laine C, Taichman DB, Mulrow C. Trustworthy clinical guidelines. Ann Intern Med 2011; 154: 774–5.
- Qaseem A, Forland F, Macbeth F, Ollenschlager G, Phillips S, van der Wees P, Board of Trustees of the Guidelines International Network. Guidelines International Network: toward international standards for clinical practice guidelines. Ann Intern Med 2012; 156: 525–31.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, FalckYtter Y, Alonso-Coello P, Schunemann HJ. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008; 336: 924–6.
- Brouwers MC, Kerkvliet K, Spithoff K, AGREE Next Steps Consortium. The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. BMJ 2016; 352: i1152.
- Jammer I, Wickboldt N, Sander M, Smith A, Schultz MJ, Pelosi P, Leva B, Rhodes A, Hoeft A, Walder B, Chew MS, Pearse RM. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: A statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol 2015; 32: 88–105.
- Guyatt GH, Oxman AD, Kunz R, Atkins D, Brozek J, Vist G, Alderson P, Glasziou P, Falck-Ytter Y, Schunemann HJ. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol 2011; 64: 395–400.
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta-analysis. J Clin Epidemiol 2008; 61: 64–75.
- Guyatt GH, Oxman AD, Kunz R, Vist GE, FalckYtter Y, Schunemann HJ. What is “quality of evidence” and why is it important to clinicians? BMJ 2008; 336: 995–8.
- Koster G, Bekema HJ, Wetterslev J, Gluud C, Keus F, van der Horst IC. Milrinone for cardiac dysfunction in critically ill adult patients: a systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Intensive Care Med 2016; 42: 1322–35.
- WHO. WHO Collaborating Centre for Drug Statistics Methodology. 2017. Available at: https:// www.whocc.no/ (accessed 28 February 2017).
- Gamper G, Havel C, Arrich J, Losert H, Pace NL, Mullner M, Herkner H. Vasopressors for hypotensive shock. Cochrane Database Syst Rev 2016; 2: CD003709.
- De Backer D, Aldecoa C, Njimi H, Vincent JL. Dopamine versus norepinephrine in the treatment of septic shock: a meta-analysis*. Crit Care Med 2012; 40: 725–30.
- Wilkman E, Kaukonen KM, Pettila V, Kuitunen A, Varpula M. Association between inotrope treatment and 90-day mortality in patients with septic shock. Acta Anaesthesiol Scand 2013; 57: 431–42.
- Alhashemi JA, Alotaibi QA, Abdullah GM, Shalabi SA. Levosimendan vs dobutamine in septic shock. J Crit Care 2009; 24: e14–5.
- Memis D, Inal MT, Sut N. The effects of levosimendan vs dobutamine added to dopamine on liver functions assessed with noninvasive liver function monitoring in patients with septic shock. J Crit Care 2012; 27: 318. e1–6.
- Morelli A, Donati A, Ertmer C, Rehberg S, Lange M, Orecchioni A, Cecchini V, Landoni G, Pelaia P, Pietropaoli P, Van Aken H, Teboul JL, Ince C, Westphal M. Levosimendan for resuscitating the microcirculation in patients with septic shock: a randomized controlled study. Crit Care 2010; 14: R232.
- Vaitsis J, Michalopoulou H, Thomopoulos C, Massias S, Stamatis P. Use of levosimendan in myocardial dysfunction due to sepsis. Crit Care 2009; 13(Suppl. 1): P165.
- Hajjej Z, Meddeb B, Sellami W, Labbene I, Morelli A, Ferjani M. Effects of levosimendan on cellular metabolic alterations in patients with septic shock: a randomized controlled pilot study. Shock 2017; 48: 307–12.
- Gordon AC, Perkins GD, Singer M, McAuley DF, Orme RM, Santhakumaran S, Mason AJ, Cross M, Al-Beidh F, Best-Lane J, Brealey D, Nutt CL, McNamee JJ, Reschreiter H, Breen A, Liu KD, Ashby D. Levosimendan for the prevention of acute organ dysfunction in sepsis. N Engl J Med 2016; 375: 1638–48.
- Mahmoud KM, Ammar AS. Norepinephrine supplemented with dobutamine or epinephrine for the cardiovascular support of patients with septic shock. Indian J Crit Care Med 2012; 16: 75–80.
- Coletta AP, Cleland JG, Freemantle N, Clark AL. Clinical trials update from the European Society of Cardiology Heart Failure meeting: SHAPE, BRING-UP 2 VAS, COLA II, FOSIDIAL, BETACAR, CASINO and meta-analysis of cardiac resynchronisation therapy. Eur J Heart Fail 2004; 6: 673–6.
- Bergh CH, Andersson B, Dahlstrom U, Forfang K, Kivikko M, Sarapohja T, Ullman B, Wikstrom G. Intravenous levosimendan vs. dobutamine in acute decompensated heart failure patients on betablockers. Eur J Heart Fail 2010; 12: 404–10.
- Duygu H, Ozerkan F, Nalbantgil S, Zoghi M, Akilli A, Akin M, Nazli C, Ergene O. Effect of levosimendan on E/E’ ratio in patients with ischemic heart failure. Int J Cardiol 2008; 123: 201–3.
- Follath F, Cleland JG, Just H, Papp JG, Scholz H, Peuhkurinen K, Harjola VP, Mitrovic V, Abdalla M, Sandell EP, Lehtonen L, Steering C. Investigators of the Levosimendan Infusion versus Dobutamine S. Efficacy and safety of intravenous levosimendan compared with dobutamine in severe low-output heart failure (the LIDO study): a randomised double-blind trial. Lancet 2002; 360: 196–202.
- Mebazaa A, Nieminen MS, Packer M, CohenSolal A, Kleber FX, Pocock SJ, Thakkar R, Padley RJ, Poder P, Kivikko M, Investigators S. Levosimendan vs dobutamine for patients with acute decompensated heart failure: the SURVIVE Randomized Trial. JAMA 2007; 297: 1883–91.
- Yontar OC, Yilmaz MB, Yalta K, Erdem A, Tandogan I. Acute effects of levosimendan and dobutamine on QRS duration in patients with heart failure. Arq Bras Cardiol 2010; 95: 738–42.
- Samimi-Fard S, Garcia-Gonzalez MJ, DominguezRodriguez A, Abreu-Gonzalez P. Effects of levosimendan versus dobutamine on long-term survival of patients with cardiogenic shock after primary coronary angioplasty. Int J Cardiol 2008; 127: 284–7.
- Garcia-Gonzalez MJ, Dominguez-Rodriguez A, Ferrer-Hita JJ, Abreu-Gonzalez P, Munoz MB. Cardiogenic shock after primary percutaneous coronary intervention: effects of levosimendan compared with dobutamine on haemodynamics. Eur J Heart Fail 2006; 8: 723–8.
- Adamopoulos S, Parissis JT, Iliodromitis EK, Paraskevaidis I, Tsiapras D, Farmakis D, Karatzas D, Gheorghiade M, Filippatos GS, Kremastinos DT. Effects of levosimendan versus dobutamine on inflammatory and apoptotic pathways in acutely decompensated chronic heart failure. Am J Cardiol 2006; 98: 102–6.
- Iyisoy A, Celik T, Celik M, Bugan B, Yaman H. Comparative effects of levosimendan and dobutamine infusion on p wave dispersion in patients with acute decompensated heart failure. Turk J Med Sci 2010; 40: 761–70.
- Yilmaz MB, Yontar C, Erdem A, Karadas F, Yalta K, Turgut OO, Yilmaz A, Tandogan I. Comparative effects of levosimendan and dobutamine on right ventricular function in patients with biventricular heart failure. Heart Vessels 2009; 24: 16–21.
- Karlsberg RP, DeWood MA, DeMaria AN, Berk MR, Lasher KP. Comparative efficacy of short-term intravenous infusions of milrinone and dobutamine in acute congestive heart failure following acute myocardial infarction. Milrinone-Dobutamine Study Group. Clin Cardiol 1996; 19: 21–30.
- De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, Defrance P, Gottignies P, Vincent JL. Comparison of dopamine and norepinephrine in the treatment of shock. N Engl J Med 2010; 362: 779–89.
- Alvarez J, Bouzada M, Fernandez AL, Caruezo V, Taboada M, Rodriguez J, Ginesta V, Rubio J, Garcia-Bengoechea JB, Gonzalez-Juanatey JR. Hemodynamic effects of levosimendan compared with dobutamine in patients with low cardiac output after cardiac surgery. Rev Esp Cardiol 2006; 59: 338–45.
- Alvarez J, Taboada M, Rodriguez J. Hemodynamic effects of levosimendan following cardiac surgery. Rev Esp Anestesiol Reanim 2005; 52: 389–94.
- Levin R, Porcile R, Salvagio F, DelMazo C, Botbol A, Tanus E, Blanco N, Degrange M. Levosimendan reduces mortality in postoperative low cardiac output syndrome after coronary surgery. J Card Surg 2009; 120: S987–8.
- Levin RL, Degrange MA, Porcile R, Salvagio F, Blanco N, Botbol AL, Tanus E, del Mazo CD. The calcium sensitizer levosimendan gives superior results to dobutamine in postoperative low cardiac output syndrome. Rev Esp Cardiol 2008; 61: 471–9.
- Mehta RH, Leimberger JD, van Diepen S, Meza J, Wang A, Jankowich R, Harrison RW, Hay D, Fremes S, Duncan A, Soltesz EG, Luber J, Park S, Argenziano M, Murphy E, Marcel R, Kalavrouziotis D, Nagpal D, Bozinovski J, Toller W, Heringlake M, Goodman SG, Levy JH, Harrington RA, Anstrom KJ, Alexander JH, Investigators L-C. Levosimendan in patients with left ventricular dysfunction undergoing cardiac surgery. N Engl J Med 2017; 376: 2032–42.
- Landoni G, Lomivorotov VV, Alvaro G, Lobreglio R, Pisano A, Guarracino F, Calabro MG, Grigoryev EV, Likhvantsev VV, Salgado-Filho MF, Bianchi A, Pasyuga VV, Baiocchi M, Pappalardo F, Monaco F, Boboshko VA, Abubakirov MN, Amantea B, Lembo R, Brazzi L, Verniero L, Bertini P, Scandroglio AM, Bove T, Belletti A, Michienzi MG, Shukevich DL, Zabelina TS, Bellomo R, Zangrillo A, CHEETAH Study Group. Levosimendan for hemodynamic support after cardiac surgery. N Engl J Med 2017; 376: 2021–31.
- Cholley B, Caruba T, Grosjean S, Amour J, Ouattara A, Villacorta J, Miguet B, Guinet P, Levy F, Squara P, Ait Hamou N, Carillon A, Boyer J, Boughenou MF, Rosier S, Robin E, Radutoiu M, Durand M, Guidon C, Desebbe O, Charles-Nelson A, Menasche P, Rozec B, Girard C, Fellahi JL, Pirracchio R, Chatellier G. Effect of levosimendan on low cardiac output syndrome in patients with low ejection fraction undergoing coronary artery bypass grafting with cardiopulmonary bypass: the LICORN randomized clinical trial. JAMA 2017; 318: 548–56.
- Feneck RO, Sherry KM,Withington PS, OduroDominah A, EuropeanMilrinoneMulticenter Trial Group. Comparison of the hemodynamic effects of milrinonewith dobutamine in patients after cardiac surgery. J Cardiothorac Vasc Anesth 2001; 15: 306–15.
- Hultcrantz M, Rind D, Akl EA, Treweek S, Mustafa RA, Iorio A, Alper BS, Meerpohl JJ, Murad MH, Ansari MT, Katikireddi SV, Ostlund P, Tranaeus S, Christensen R, Gartlehner G, Brozek J, Izcovich A, Schunemann H, Guyatt G. The GRADE Working Group clarifies the construct of certainty of evidence. J Clin Epidemiol 2017; 87: 4–13.
- Savovic J, Jones HE, Altman DG, Harris RJ, Juni P, Pildal J, Als-Nielsen B, Balk EM, Gluud C, Gluud LL, Ioannidis JP, Schulz KF, Beynon R, Welton NJ, Wood L, Moher D, Deeks JJ, Sterne JA. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Ann Intern Med 2012; 157: 429–38.
- Landoni G, Comis M, Conte M, Finco G, Mucchetti M, Paternoster G, Pisano A, Ruggeri L, Alvaro G, Angelone M, Bergonzi PC, Bocchino S, Borghi G, Bove T, Buscaglia G, Cabrini L, Callegher L, Caramelli F, Colombo S, Corno L, Del Sarto P, Feltracco P, Forti A, Ganzaroli M, Greco M, Guarracino F, Lembo R, Lobreglio R, Meroni R, Monaco F, Musu M, Pala G, Pasin L, Pieri M, Pisarra S, Ponticelli G, Roasio A, Santini F, Silvetti S, Szekely A, Zambon M, Zucchetti MC, Zangrillo A, Bellomo R. Mortality in multicenter critical care trials: an analysis of interventions with a significant effect. Crit Care Med 2015; 43: 1559–68.
- Perner A, Myburgh J. Ten ‘short-lived’ beliefs in intensive care medicine. Intensive Care Med 2015; 41: 1703–6.
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso-Coello P, Atkins D, Kunz R, Brozek J, Montori V, Jaeschke R, Rind D, Dahm P, Meerpohl J, Vist G, Berliner E, Norris S, Falck-Ytter Y, Murad MH, Schunemann HJ. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol 2011; 64: 1311–6.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Appendix S1. Trial sequential analyses.
Fig. S1. Trial sequential analysis of dobutamine vs. other inotropes in patients with septic shock.
Fig. S2. Trial sequential analysis of dobutamine vs. other inotropes in patients with cardiogenic shock.
Fig. S3. Trial sequential analysis of dobutamine vs. other inotropes in patients with shock after cardiac surgery.
Appendix S2. GRADE summary of findings tables.
Table S1. Summary of findings for patients with shock in general.
Table S2. Summary of findings for patients with septic shock.
Table S3. Summary of findings for patients with cardiogenic shock.
Table S4. Summary of findings for patients with hypovolemic shock.
Table S5. Summary of findings for patients with shock after cardiac surgery.
Table S6. Summary of findings for patients with other types of shock, including vasodilatory shock. Appendix S3. Conflicts of interest on a recommendation per recommendation basis per coauthor.
Appendix S3. Conflicts of interest on a recommendation per recommendation basis per coauthor.
