Endorsement af ERC´s afsnit om hjertestop hos gravide:
Truhlář A, Deakin CD, Soar J et al. European Resuscitation Council Guidelines for Resuscitation 2015: Section 4. Cardiac arrest in special circumstances. Resusicitation. 2015 Oct; 95:148-201.
Af DASAIM´s Obstetrisk anæstesiudvalg
Revideret april 2023
Fysiologiske ændringer under graviditeten:
- Øget blodvolumen og cardiac output
- Øget minutventilation og ilt-forbrug
- Kompression af v. cava inferior kan medføre nedsat cardiac output og blodtryk
- Ødem af øvre luftveje
- Refluks
Hyppigste direkte årsager til død under graviditeten i I-lande:
- Blødning
- Emboli (trombotisk el amnionemboli)
- Hypertensive tilstande (herunder præeclampsi),
- Abort
- Sepsis
Tilgang til den kritisk syge gravide:
- Generel brug af ABCDE
- Venstre sideleje el. manuel displacering af uterus mod venstre
- Iltterapi vejledt af saturationsmåling
- Giv væskebolus ved hypotension eller tegn på hypovolæmi
- Reevaluer indikation for evt. medicin
- Tidligt tilsyn af pædiater og obstetriker
- Aggressiv behandling af til grundlæggende årsag
Basal hjerte-lungeredning ved hjertestop:
- Pædiater og obstetriker kaldes tidligt
- Kompressioner og ventilationer iflg. standard 30:2
- Høj kvalitet af hjertemassagen med minimale afbrydelser
- Evt. kompressioner højere på sternum sidst i graviditeten
- Cava-kompression aflastes med manuel displacering af uterus mod venstre (alternativt 15-30 graders venstre tilt, fx med pude under hoften)
Avanceret behandling af hjertestop modificeret hos gravide:
- Defibrillering efter gældende voksne retningslinjer
- Evt. tidlig intubation grundet aspirationsrisiko og øget intraabdominalt tryk
- Endotrachealtube 0,5-1 mm ID mindre end til ikke-gravid grundet luftvejsødemer
- Grundet risiko for vanskelig intubation kan opstå behov for ekspert-hjælp, nødprocedure og alternativt luftvejsudstyr
- Om muligt i.v. anlæggelse over v. cava inf.
- Reversible årsager: I tillæg til ”4 H´er og 4 T´er” overvejes:
- Blødning: Overvej infusionssystem til hurtig blodtransfusion og brug af autotransfusion (cell salvage)
- AMI: Overvej reperfusionsbehandling med PCI el. trombolyse
- Peripartum kardiomypati
- Lungeemboli: Overvej trombolyse. Amnion emboli behandles derimod understøttende
- Præeklampsi/ eklampsi: Magnesiumsulfat som krampeprofylakse og behandling
- Perimortem sectio fra og med 20. graviditetsuge:
- På hospital: forbered perimortem sectio (tilkald obstetrisk læge og neonatolog/pæd). Indiceret inden 5 minutters hjertestop mhp. maximal cavaaflastning
- 20.-23.uge: Barnets overlevelse usandsynlig
- ≥ 24. uge: Barnets overlevelse mulig
- Præhospital vil perimortem sectio kun ekstremt sjældent være indiceret.
- Præhospitalt perimortem sectio vil kræve at den præhospital læge er oplært i indgrebet og disse kompetencer vedligeholdes. Og at den præhospitale læge skønner det indiceret og meningsfyldt.
Avanceret behandling efter hjertestop: Ingen modifikationer i forhold til ikke-gravide
Anbefalede organisatoriske tiltag:
- Planer for og udstyr til maternel og neonatal genoplivning
- Tidlig alarmering af obstetriske, pædiatriske og anæstesiologiske teams
- Regelmæssig træning i akutte obstetriske situationer
European Resuscitation Council Guidelines for Resuscitation 2015 Section 4.
Cardiac arrest in special circumstances
AnatolijTruhlárˇa, b, ∗, CharlesD.Deakinc, JasmeetSoard , GamalEldinAbbasKhalifae, AnnetteAlfonzof, JoostJ.L.M.Bierensg, GuttormBrattebøh, HermannBruggeri, JoelDunningj, SilvijaHunyadi-Anticeviˇc´k, RudolphW.Kosterl, DavidJ.Lockeym, w, CarstenLottn , PeterPaal o,p, GavinD.Perkinsq,r, ClaudioSandronis,Karl-ChristianThiest, DavidA.Zidemanu, JerryP.Nolanv,w, on behalf of the Cardiac arrest in special circumstances section Collaborators1
a Emergency Medical Services of the Hradec Králové Region, Hradec Králové, Czech Republic
b Department of Anaesthesiology and Intensive Care Medicine, University Hospital Hradec Králové, Hradec Králové, Czech Republic
c Cardiac Anaesthesia and Cardiac Intensive Care, NIHR Southampton Respiratory Biomedical Research Unit, Southampton University Hospital NHS Trust, Southampton,UK
d Anaesthesia and Intensive Care Medicine, Southmead Hospital, North Bristol NHS Trust, Bristol, UK
e Emergency and Disaster Medicine, Six October University Hospital, Cairo, Egypt
f Departments of Renaland Internal Medicine, Victoria Hospital, Kirkcaldy, Fife, UK
g Society to Rescue People from Drowning, Amsterdam, The Netherlands
h Bergen Emergency Medical Services, Department of Anaesthesia and Intensive Care, Haukeland University Hospital, Bergen,Norway
i EURAC Institute of Mountain Emergency Medicine, Bozen, Italy
j Department of Cardiothoracic Surgery, James Cook University Hospital, Middlesbrough, UK
k CenterforEmergencyMedicine,ClinicalHospitalCenter Zagreb,Zagreb,Croatia
l Department of Cardiology, Academic Medical Center, Amsterdam, The Netherlands
m Intensive Care Medicine and Anaesthesia, Southmead Hospital, NorthBristol NHS Trust, Bristol,UK
n Department of Anesthesiology, University Medical Center, Johannes Gutenberg-Universitaet, Mainz, Germany
o Barts Heart Centre, St Bartholomew’s Hospital, Barts Health NHS Trust, Queen Mary University of London, London, UK
p Department of Anaesthesiology and Critical Care Medicine,University HospitalInnsbruck,Austria
q Warwick Medical School, University of Warwick, Coventry,UK
r Critical Care Unit,Heart of England NHS FoundationTrust,Birmingham,UK
s Department of Anaesthesiology and Intensive Care, Catholic University School of Medicine, Rome, Italy
t Birmingham Children’s Hospital, Birmingham, UK
u Department of Anaesthetics, Imperial College Healthcare NHS Trust, London, UK
v Anaesthesia and Intensive Care Medicine, Royal United Hospital, Bath, UK
w School of Clinical Sciences, University of Bristol, UK
Introduction
Irrespective of the cause of cardiac arrest, early recognition and calling for help, including appropriate management of the deteriorating patient, early defibrillation, high-quality cardiopulmonary resuscitation (CPR) with minimal interruption of chest compressions and treatment of reversible causes, are the most important interventions. In certain conditions, however, advanced life support(ALS) guidelines require modification.The following guidelines for resuscitation in special circumstances are divided into three parts:
- Corresponding author.
- E-mailaddress: anatolij.truhlar@gmail.com(A.Truhlár).ˇ
1 The members of the Cardiac arrest in special circumstances section Collaborators are listed in the Collaboratorssection.
special causes, special environments and special patients. The first part covers treatment of potentially reversible causes of cardiac arrest, for which specific treatment exists, and which must be identified or excluded during any resuscitation. For improving recall during ALS, these are divided into two groups of four, based upon their initial letter– either H or T– and are called the ‘4Hs and 4Ts’:Hypoxia; Hypo-/hyperkalaemia and other electrolyte disorders; Hypo-/hyperthermia; Hypovolaemia; Tension pneumothorax; Tamponade (cardiac); Thrombosis (coronary and pulmonary); Toxins (poisoning). The second part covers cardiac arrest in special environments, where universal guidelines have to be modified due to specific locations or location-specific causes of cardiac arrest.The third part is focused on patients with specific conditions, and those with certain long-term comorbidities where a modified approach and different treatment decisions may be necessary.
Summary of changes since 2010 Guidelines
The main changes in the ERC Guidelines 2015 in comparison with the Guidelines 20101 are summarised below:
Special causes
- Survival after an asphyxia-induced cardiac arrest is rare and survivors often have severe neurological impairment.During CPR, early effective ventilation of the lungs with supplementary oxygen is essential.
- A high degree of clinical suspicion and aggressive treatment can prevent cardiac arrest from electrolyte abnormalities. The new algorithm provides clinical guidance to emergency treatment of life-threatening hyperkalaemia.
- Hypothermic patients without signs of cardiac instability (systolic blood pressure ≥90 mmHg, absence of ventricular arrhythmias or core temperature ≥28◦C) can be rewarmed externally using minimally invasive techniques (e.g. with warm forced air and warm in travenous fluid).Patients with signs of cardiac in stability should be transferred directly to a centre capable of extra corporeal life support(ECLS).
- Early recognition and immediate treatment with intramuscular adrenaline remains the main stay of emergency treatment for anaphylaxis.
- The mortality from traumatic cardiac arrest (TCA) is very high. The most common cause of death is haemorrhage. It is recognised that most survivors do not have hypovolaemia, but instead have other reversible causes(hypoxia, tension pneumothorax, cardiac tamponade) that must be immediately treated. The new treatment algorithm for TCA was developed to prioritise the sequence of lifesaving measures. Chest compressions should not delay the treatment of reversible causes. Cardiac arrests of non-traumatic origin leading to a secondary traumatic event should be recognised and treated with standard algorithms.
- There is limited evidence for recommending the routine transport of patients with continuing CPR after out-of-hospital cardiac arrest(OHCA)of suspected cardiac origin. Transport may be beneficial in selected patients where there is immediate hospital access to the catheterisation laboratory and an infrastructure providing prehospital and in-hospital teams experienced in mechanicalor haemodynamic support and percutaneous coronary intervention(PCI) with ongoing CPR.
- Recommendations for administration of fibrinolytics when pulmonary embolism is the suspected cause of cardiac arrest remain unchanged. Routine use of surgical embolectomy or mechanical thrombectomy when pulmonary embolism is the suspected cause of cardiac arrest is not recommended. Consider these methods only when there is a known diagnosis of pulmonary embolism.
- Routine use of gastric lavage for gastrointest in aldecontamination in poisoning is no longer recommended. Reduced emphasis is placed on hyperbaric oxygen therapy in carbon monoxide poisoning.
Special environments
- The special environments section includes recommendations for treatment of cardiac arrest occurring in specific locations. These locations are specialised healthcare facilities (e.g. operating theatre, cardiac surgery, catheterisation laboratory, dialysis unit, dental surgery), commercial airplanes or air ambulances, field of play, outside environment (e.g.drowning, difficult terrain, high altitude, avalanche burial, lightning strike and electrical injuries) or the scene of a mass casualty incident.
- Patients undergoing surgical procedures involving general anaesthesia, particularly in emergencies, are at risk from perioperative cardiac arrest. A new section covers the common causes and relevant modification to resuscitative procedures in this group of patients.
- Cardiac arrest following major cardiac surgery is relatively common in the immediate post-operative phase. Key to successful resuscitation is recognition of the need to perform emergency resternotomy, especially in the context of tamponade or haemorrhage, where external chest compressions may be ineffective. Resternotomy should be performed within 5 min if other interventions have failed.
- Cardiac arrest from shockable rhythms (Ventricular Fibrillation (VF) or pulselessVentricularTachycardia(pVT)) during cardiac catheterisation should immediately be treated with up to three stacked shocks before starting chest compressions. Use of mechanical chest compression devices during angiography is recommended to ensure high-quality chest compressions and reduce the radiation burden to personnel during an gio graphy with ongoing CPR.
- In dental surgery, do not move the patient from the dental chair in order to start CPR.Quickly recline the dental chair into a horizontal position and place a stool under the head of the chair to increase its stability during CPR.
- The in-flight use ofAEDs aboard commercial airplanes can result in up to 50% survival to hospital discharge. AEDs and appropriate CPR equipment should be mandatory onboard of all commercial aircraft in Europe, including regional and low-cost carriers. Consider an over-the-head technique of CPR if restricted access precludes a conventional method, e.g. in the aisle.
- The incidence of cardiac arrest on board helicopter emergency medical services (HEMS) and air ambulances is low. Importance of pre-flight preparation and use of mechanical chest compression devices are emphasised.
- Sudden and unexpected collapse of an athlete on the field of play is likely to be cardiac in origin and requires rapid recognition and early defibrillation.
- The duration of submersion is a key determinant of outcome from drowning. Submersion exceeding 10 min is associated with poor outcome. Bystanders play a critical role in early rescue and resuscitation.Resuscitation strategies for those in respiratory or cardia carrest continue to prioritise oxygenation and ventilation.
- The chances of good outcome from cardiac arrest in difficult terrain or mountains may be reduced because of delayed access and prolonged transport. There is a recognised role of air rescue and availability of AEDs in remote but often-visited locations.
- The cut-off criteria for prolonged CPR and extracorporeal rewarming of avalanche victims in cardiac arrest are more stringent to reduce the number of futile cases treated with extra corpoereal lifesupport(ECLS). ECLS is indicated if the duration of burial is >60 min (instead of >35 min), core temperature at extrication is <30◦C (instead of <32◦C), and serum potassium at hospital admissionis ≤8 mmolL− 1 (instead of ≤12mmolL− 1 ); otherwise standard guidelines apply.
- Safety measures are emphasised when providing CPR to the victim of an electrical injury.
- Recommendations for management of multiple victims should prevent delay of treatment available for salvageable victims during mass casualty incidents (MCIs). Safety at scene is paramount. A triage system should be used to prioritise treatment and, if the number of casualties overwhelms healthcare resources, withhold CPR for those without signs of life.
Special patients
- The section on special patients gives guidance forCPR in patients with severe comorbidities (asthma, heart failure with ventricular assist devices, neurological disease, obesity) and those with specific physiological conditions(pregnancy, elderly people).
- The first line treatment for acute asthma is inhaled beta-2 agonists while intravenous beta-2 agonists are suggested only for those patients in whom inhaled therapy cannot be used reliably. Inhaled magnesium is no longer recommended.
- In patients with ventricular assist devices(VADs), confirmation of cardiac arrest may be difficult. If during the first 10 days after surgery, cardiac arrest does not respond to defibrillation, perform resternotomy immediately.
- Patients with subarachnoid haemorrhage may have ECG changes that suggest an acute coronary syndrome (ACS). Whether a computed tomography (CT) brain scan is done before or after coronary angiography will depend on clinical judgement regarding the likelihood of a subarachnoid haemorrhage versusacute coronary syndrome. • No changes to these quence of actions are recommended in resuscitation of obese patients, although delivery of effective CPR may be challenging. Consider changing rescuers more frequently than the standard 2-min interval. Early tracheal intubation by an experienced provider is recommended.
- For the pregnant woman in cardiac arrest, high-quality CPRwith manualuterine displacement, early ALS and delivery of the fetus if early return of spontaneous circulation(ROSC) is not achieved remain key interventions.
A–SPECIAL CAUSES
Hypoxia
Introduction
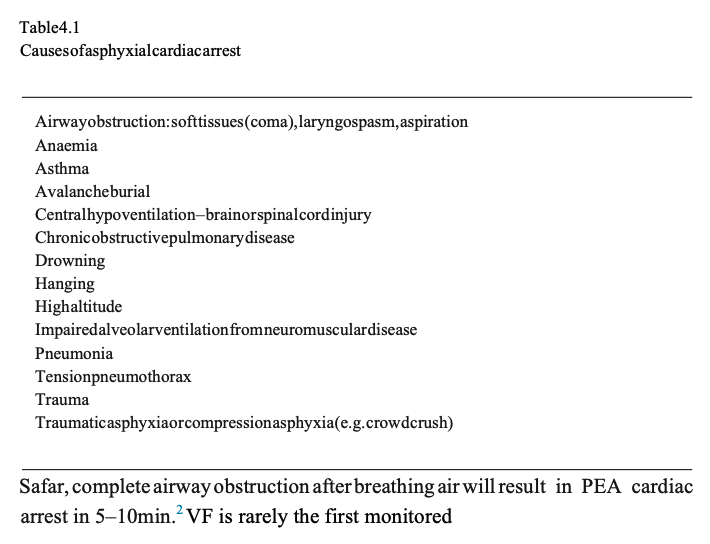
Cardiac arrest caused by pure hypoxaemia is uncommon. It is seen more commonly as a consequence of asphyxia, which accounts for most of the noncardiac causes of cardiac arrest.There are many causes of asphyxial cardiac arrest (Table 4.1); although there is usually a combination of hypoxaemia and hypercarbia, it is the hypoxaemia that ultimately causes cardiac arrest.2
Pathophysiological mechanisms
If breathing is completely prevented by airway obstruction or apnoea, consciousness will be lost when oxygens aturation in the arterial blood reaches about 60%.The time taken to reach this concentration is difficult to predict, but is likely to be of the order 1–2min.3 Based on animal experiments of cardiac arrest caused by asphyxia, pulseless electrical activity (PEA) will occur in 3–11min. A systole will ensue several minutes later.4 In comparison with simple apnoea, the exaggerated respiratory movements that frequently accompany airway obstruction will increase oxygen consumption resulting in more rapid arterial blood oxygen desaturation and a shorter time to cardiac arrest. According to rhythm after asphyxial cardiac arrest – in one of the largest series of hanging associated out-of-hospital cardiac arrests (OHCAs),from Melbourne, Australia, just 7 (0.5%) of 1321 patients were in VF.5
Treatment
Treating the cause of the asphyxia/hypoxaemia is the highest priority because this is a potentially reversible cause of the cardiac arrest. Effective ventilation with supplementary oxygen is a particular priority in these patients. The better outcomes for OHCA victims receiving compression-only CPR6 is not the case for as phyxial cardiac arrests, which have much better survival rates with conventional CPR.7 Follow the standard ALS algorithm when resuscitating these patients.
Outcome
Survival after cardiac arrest from asphyxia is rare and most survivors sustain severe neurological injury. Of five published series that included a total of 286 patients with cardiac arrest following hanging where CPR was attempted (this was attempted in only about 16% of cases), there were just six (2%) survivors with a full recovery;11 other survivors all had severe permanent brain injury.5, 8–11 In one third (89; 31%) of these 286 patients, rescuers were able to achieve ROSC–thus when CPR is attempted, ROSC is not uncommon but subsequent neurologically intact survival is rare. Those who are unconscious but have not progressed to a cardiac arrest are much more likely to make a good neurological recovery.11, 12
Hypo-/hyperkalaemia and other electrolyte disorders
Introduction
Electrolyte abnormalities can cause cardiac arrhythmias or cardiac arrest. Lifethreatening arrhythmias areas sociated most commonly with pot assium disorders, particularly hyperkalaemia, and less commonly with disorders ofserum calcium and magnesium. Consider electrolyte disturb ances in patient groups at risk–renal failure, severe burns, cardiac failure and diabetes mellitus. The electrolyte values for definitions have been chosen as a guide to clinical decision-making.The precise values that trigger treatment decisions will depend on the patient’s clinical condition and rate of change of electrolyte values. There is little or no evidence for the treatment of electrolyte abnormalities during cardiac arrest. Guidance during cardiac arrest is based on the strategies used in the non-arrest patient.
Prevention of electrolyte disorders
When possible, identify and treat life-threatening electrolyte abnormalities before cardiac arrest occurs. Monitor renal function in patients at risk and avoid combination of drugs that may exacerbate hyperkalaemia. Prevent recurrence of electrolyte disorders by removing any precipitating factors(e.g. drugs, diet).
Potassium disorders
Potassium homeostas is. Extra cellular potassium concentration is regulated tightly between 3.5 and 5.0 mmolL− 1. A large concentration gradient normally exists between intracellular and extracellular fluid compartments.This potassiumgradient across cell membranes contributes to the excitability of nerve and muscle cells, including the myocardium. Evaluation of serumpotassium musttake into consideration the effects of changes in serum pH. When serum pH decreases (acidaemia), serum potassium increases because potassium shifts from the cellular to the vascular space; a process that is reversed when serum pH increases (alkalaemia). Hyperkalaemia. This is the most common electrolyte disorder associated with cardiac arrest. It is usually caused by impaired excretion by the kidneys, drugs or increased potassium release from cells and metabolic acidosis. Hyperkalaemia occurs in up to 10%of hospitalised patients.13–15 Chronic kidney disease (CKD) is common in the general population and the incidence of hyperkalaemia increases from 2 to 42% as glomerular filtration rate(GFR)drops from 60 to 20m L min− 1.16 Patients with end-stage renal disease are particularly susceptible, particularly following an OHCA.17 Prolonged hyperkalaemia is an independent risk factor for in-hospital mortality.18 Acute hyperkalaemia is more likely than chronic hyperkalaemia to cause life-threatening cardiac arrhythmiasor cardiac arrest. Definition. There is no universal definition. We have defined hyperkalaemia as a serum potassium concentration higher than 5.5 mmolL− 1; in practice, hyperkalaemia is a continuum. As the potassium concentration increases above this value the risk of adverse events increases and the need for urgent treatment increases. Severe hyperkalaemia has been defined as a serum potassium concentration higher than 6.5 mmolL− 1.
Causes. The main causes of hyperkalaemia are:
- renal failure(i.e. acute kidney injury or chronic kidney disease);
- drugs (e.g. angiotensin converting enzyme inhibitors (ACE-I), angiotensin II receptor antagonists (ARB), potassium-sparing diuretics, non-steroidal anti inflammatory drugs, beta-blockers, trimethoprim);
- tissue breakdown (e.g.rhabdomyolysis, tumourlysis, haemolysis);
- metabolic acidosis (e.g. renal failure, diabetic keto acidosis);
- endocrine disorders (e.g. Addison’s disease);
- diet(may be sole cause in patients with advanced chronic kidney disease) and
- spurious–pseudo-hyperkalaemia (suspect in cases with normal renal function, normal ECG and/or history of haematological disorder). Pseudo-hyperkalaemia describes the finding of a raised serum (clotted blood) K+ value concurrently with a normal plasma (non-clotted blood) potassium value. The clotting process releases K+ from cells and platelets, which increases the serum K+ concentration by an average of 0.4mmol/L. The most common cause of pseudo-hyperkalaemia is a prolonged transittime to the laboratory or poor storage conditions.19, 20
The risk of hyperkalaemia is even greater when there is a combination of factors such as the concomitant use of angiotensinconverting enzyme inhibitors or angiotens in II receptor blockers and potassium-sparing diuretics.
Recognition of hyperkalaemia. Exclude hyperkalaemia in all patientswith an arrhythmia or cardiac arrest. Patients may present with weakness progressing to flaccid paralysis, paraesthesia, or depressed deep tendon reflexes. Alternatively, the clinical picture can be overshadowed by the primary illness causing hyperkalaemia. The first indicator of hyperkalaemia may also be the presence of ECG abnormalities, arrhythmias, or cardiac arrest. The use of a blood gas analyser to measure potassium can reduce delays in recognition.21, 22
The effect of hyperkalaemia on the ECG depends on the absolute serum potassium as well as the rate of increase.23 The reported frequency of ECG changes in severe hyperkalaemia is variable, but most patients appear to show ECG abnormalities at a serum potassium concentration higher than 6.7 mmolL− 1. 23, 24 The presence of ECG changes strongly correlates with mortality.25 In some cases, the ECG may be normal or show a typical changes including ST elevation. The ECG changes associated with hyperkalaemia are usually progressive and include:
- first degree heartblock (prolonged PR interval>0.2s);
- flattened or absent P waves;
- tall, peaked (tented) T waves(i.e. Twave larger than R wave in more than 1 lead);
- ST-segment depression;
- S&T wave merging(sine wave pattern);
- widened QRS(>0.12s);
- ventricular tachy cardia;
- bradycardia;
- cardiac arrest (PEA, VF/pVT, asystole).
Treatment of hyperkalaemia. There are five key treatment strategies for hyperkalaemia22:
- cardiac protection;
- shifting potassium into cells;
- removing potassium from the body;
- monitoring serum potassium and blood glucose;
- prevention of recurrence.
When hyperkalaemia is strongly suspected, e.g. in the presence of ECG changes, start life-saving treatment even before laboratory results are available. The treatment strategy for hyperkalaemia has been reviewed extensively.13, 22, 26 Follow the hyperkalaemia emergency treatment algorithm (Fig. 4.1).22 Avoid salbutamol monotherapy, which may be ineffective. There is insufficient evidence to support the use of sodium bicarbonate to decrease serum potassium. Consider the need for early specialist or critical care referral.
The main risks associated with treatment of hyperkalaemia are:
- Hypoglycaemia following insulin-glucose administration (usually occurs within 1–3 hof treatment, but may occur up to 6h after infusion).27 Monitor blood glucose and treat hypoglycaemia promptly.
- Tissue necrosis secondary to extravasation of intravenous calciumsalts. Ensure secure vascular access prior to administration.
- Intestinal necrosis or obstruction following use of potassium exchange resins. Avoid prolonged use of resins and give laxative.
- Rebound hyperkalaemia after the effect of drug treatment has worn off (i.e.within4–6h). Continue to monitor serum potassium for a minimum of 24h after an episode. Patient not in cardia carrest Assess patient:
- Use systematic ABCDE approach and correct any abnormalities, obtain IV access.
- Check serum potassium.
- Record an ECG. Monitor cardiac rhythm in patients with severe hyperkalaemia. Treatment is determined according to severity of hyperkalaemia. Approximate values are provided to guide treatment. Follow hyperkalaemia emergency treatment algorithm (Fig.4.1).
- Mild elevation (5.5–5.9 mmol L− 1).
- Address cause of hyperkalaemia to correct and avoid further rise in serum potassium(e.g. drugs, diet).
- If treatment is indicated, remove potassium from the body: potassium exchange resins-calcium resonium 15–30g, or sodium polystyrenesulfonate (Kayexalate) 15–30g, given either or all yor by retentionenema/PR(perrectum)(on setin >4h).
- Moderate elevation (6.0–6.4 mmol L− 1) without ECG changes.
- Shift potassium in tracellularly with glucose/insulin: 10 units short-acting insulin and 25g glucose IVover 15–30min(onset in15–30min; maximal effect at 30–60 min; duration of action 4–6h; monitor blood glucose).
- Remove potassium from the bod y(see above; consider dialysis guided by clinical setting). Reproduced with permission from Renal Association and Resuscitation Council(UK). Severe elevation(≥6.5m molL− 1) without ECG changes.
- Seek expert help.
- Give glucose/insulin(see above).
- Gives albutamol 10–20 mgnebulised (on setin 15–30min; duration of action 4–6 h)
- Remove potassium from the body (consider dialysis). Severe elevation(≥6.5m molL− 1) with toxic ECG changes.
- Seek expert help.
- Protect the heart with calcium chloride: 10 mL 10% calcium chloride IVover 2–5 min to antagonise the toxic effects of hyperkalaemia at the myocardial cell membrane. This protects the heart by reducing the risk of VF/pVT but does not lower serum potassium(on setin 1–3min).
- Use shifting agents (glucose/insulin and salbutamol).
- Remove potassium from the body (consider dialysis at out setor if refractory to medical treatment). Modifications to cardio pulmonary resuscitation.The following modifications to standard ALS guidelines are recommended in the presence of severe hyperkalaemia:
- Confirm hyperkalaemia using a blood gas analyser if available.
- Protect the heart: give 10mL calcium chloride 10% IV by rapid bolus injection.
- Shift potassium in to cells: Give glucose/insulin: 10 units short acting insulin and 25g glucose IV by rapid injection. Monitor blood glucose.
- Give sodium bicarbonate: 50 mmol IV by rapid injection (if severe acidosis or renal failure).
- Remove potassium from body: Consider dialysis for hyperkalaemic cardiac arrest resistant to medical treatment. Several dialysis modalities have been used safely and effectively in cardiac arrest, but this may only be available in specialist centres.28

Fig.4.1. Emergency treatment of hyperkalaemia. PR perrectum; ECG electro cardiogram; VT ventricular tachycardia. Reproduced with permission from Renal Association and Resuscitation Council(UK).
Consider use of a mechanical chest compression device if prolonged CPR is needed.
Indications for dialysis. The main indications for dialysis in patients with hyperkalaemia are:
- severe life-threatening hyperkalaemia with or without ECG changes or arrhythmia;
- hyperkalaemia resistant to medical treatment
- end-stage renal disease;
- oliguric acute kidney injury (<400 mL day − 1 urine output);
- marked tissue breakdown (e.g.rhabdomyolysis). Special considerations for management of cardiac arrest in a dialysis unit are addressed in the section
Special environments (see cardiac arrest in a dialysis unit). Hypokalaemia. Hypokalaemia is the most common electrolyte disorder in clinical practice.29 It is seen in up to 20% of hospitalised patients.30 Hypokalaemia increases the incidence of arrhythmias and sudden cardiac death (SCD).31 The risk is increased in patients with pre-existing heart disease and in those treated with digoxin.
Definition. Hypokalaemia is defined as a serum potassium level <3.5m molL− 1. Severe hypokalaemia is defined as a serum potassium level <2.5m molL− 1 and may be associated with symptoms.
Causes. The main causes of hypokalaemia include:
- gastro in testinal loss (e.g.diarrhoea);
- drugs (e.g. diuretics, laxatives, steroids);
- renal losses(e.g. renal tubular disorders, diabetes in sipidus, dialysis);
- endocrine disorders (e.g. Cushing’s syndrome, hyperaldosteronism);
- metabolical kalosis;
- magnesium depletion;
- poor dietary intake.
Treatment strategies used for hyperkalaemia may also induce hypokalaemia.
Recognition of hypokalaemia. Exclude hypokalaemia in every patient with an arrhythmia or cardiac arrest. In dialysis patients, hypokalaemia may occur at the end of a haemo dialysis session or during treatment with peritoneal dialysis.
As serum potassium concentration decreases, the nerves and muscles are predominantly affected, causing fatigue, weakness, leg cramps, constipation. In severe cases (serum potassium <2.5m molL− 1), rhabdomyolysis, ascending paralysis and respiratory difficulties may occur.
ECG features of hypokalaemia are:
- Uwaves;
- Twave flattening;
- ST segment changes;
- arrhythmias, especially if patient is taking digoxin;
- cardiac arrest(PEA, VF/pVT, asystole).
Treatment.This depends on the severity of hypokalaemia and the presence of symptoms and ECG abnormalities. Gradual replacement of potassium is preferable, but in an emergency, intravenous potassium is required. The maximum recommended IV dose of potassium is 20m mol h− 1, but more rapid infusion (e.g. 2m mol min− 1 for 10min, followed by 10m mol over 5–10 min) is indicated for unstable arrhythmias when cardiac arrest is imminent. Continuous ECG monitoring is essential during IV infusion and the dose should be titrated after repeated sampling of serum potassium levels.
Many patients who are potassium deficient are also deficient in magnesium. Magnesium is important for potassium uptake and for the maintenance of intracellular potassium values, particularly in the myocardium. Repletion of magnesium stores will facilitate more rapid correction of hypokalaemia and is recommended in severe cases of hypokalaemia.32
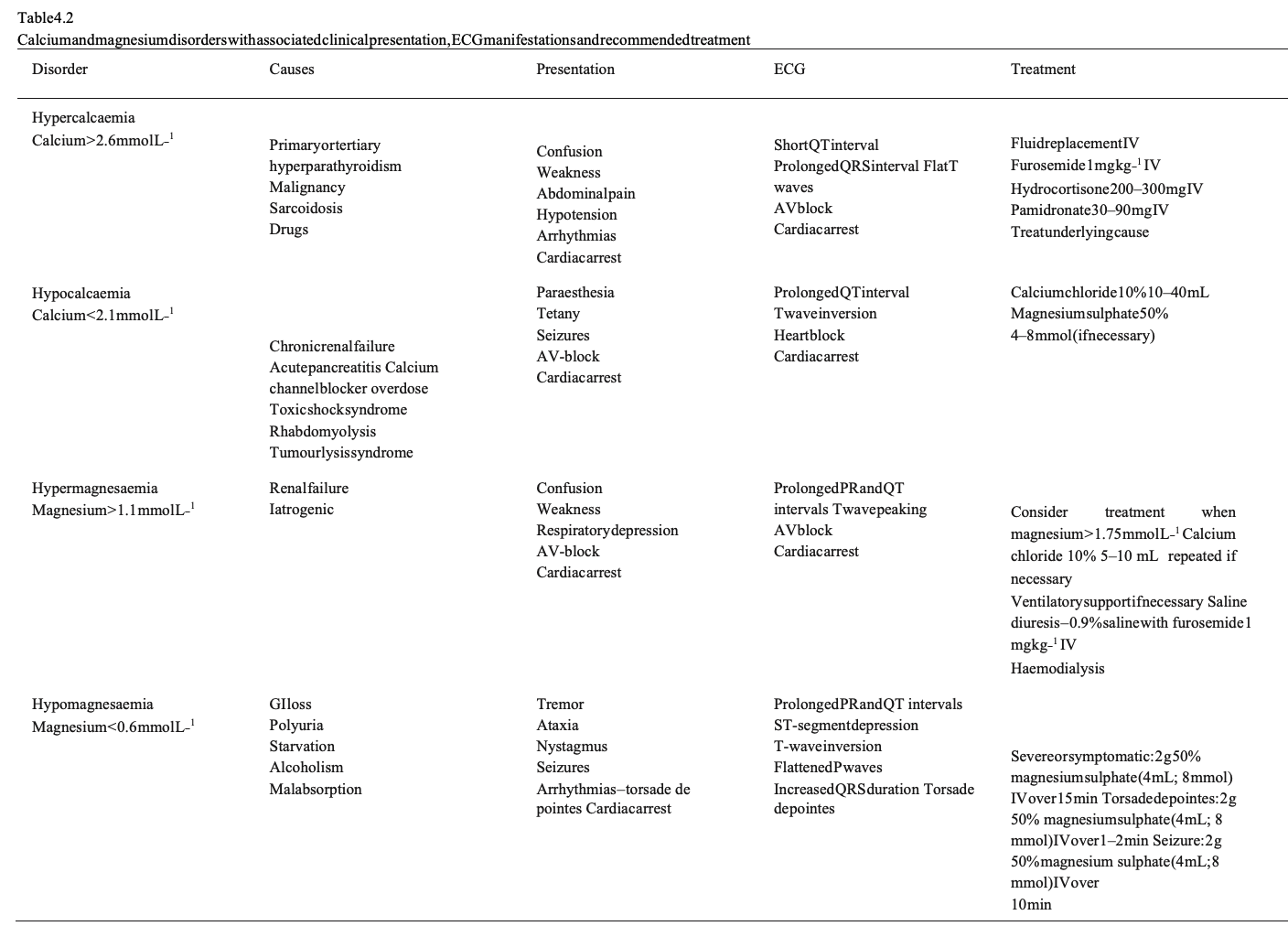
Calcium and magnesium disorders
The recognition and management of calcium and magnesium disorders is summarised in Table 4.2. Hypo-/hyperthermia Accidental hypothermia Definition. Every year approximately 1500 people die of primary accidental hypothermia in the United States.33 Accidental hypothermia is defined as an involuntary drop of the bodycore temperature<35◦C. The Swiss staging system is used to estimate core temperature at the scene.
Its stages are based on clinical signs, which roughly correlate with the core temperature:
- hypothermia I; mild hypothermia (conscious, shivering, core temperature 35– 32◦C);
- hypothermia II; moderate hypothermia (impaired consciousness without shivering, core temperature 32–28◦C);
- hypothermia III; severe hypothermia (unconscious, vitals signs present, core temperature 28–24◦C);
- hypothermia IV; cardiac arrest or low flow state (no or minimal vital signs, core temperature<24◦C);
- hypothermia V; death due to irreversible hypothermia (core temperature <13.7◦C).34
Diagnosis. Hypothermia is diagnosed in any patient with a core temperature<35◦C, or where measurement unavailable, a history of exposure to cold, or when the trunk feels cold.33 Accidental hypothermia may be under-diagnosed in countries with a temperate climate. When thermo regulation is impaired, for example, in the elderly and very young, hypothermia may follow a mild insult. The risk of hypothermia is increased by alcohol or drug ingestion, exhaustion, illness, injury or neglect especially when there is a decrease in the level of consciousness. A low-reading thermometer is needed to measure the core temperature and confirm the diagnosis. The core temperature in the lower third of the oesophagus correlates well with heart temperature. Tympanic measurement using a thermistor technique is a reliable alternative but may be considerably lower than core temperature if the environment is very cold, the probe is not well insulated, orthe external auditory canal is filled with snow or water35, 36 Widely available tympanic thermometers based on infrared technique do not seal the ear canal and are not designed for low core temperature readings.37 The in-hospital core temperature measurement site should be the same throughout resuscitation and rewarming. Bladder and rectal temperature slag behind core temperature;38, 39 for this reason, measurement of bladder and rectal temperature has been de-emphasised in patients with severe hypothermia. Decision to resuscitate. Cooling of the human body decreases cellular oxygen consumption by about 6% per 1◦C decreasein core temperature.40 At 28◦C, oxygen consumption is reduced by approximately 50% and at 22◦C by approximately 75%. At 18◦C the brain can tolerate cardiac arrest for up to 10 times longer than at 37◦C. This results in hypothermia exerting a protective effect on the brain and heart,41 and intact neurological recovery may be possible even after prolonged cardiac arrest if deep hypothermia develops before as phyxia. Beware of diagnosing death in a hypothermic patient because hypothermia itself may produce a very slow, small-volume, irregular pulse and unrecordable blood pressure. In a deeply hypothermic patient (hypothermiaIV) signs of life may be sominimal that it is easy to overlook them. Therefore, look for signs of life forat least 1 min and use an ECG monitor to detect any electrical cardiac activity. Neurologically intact survival has been reported after hypothermic cardiac arrest with a core temperature as low as 13.7◦C42 and CPR for as long as six and a half hours.43
Intermittent CPR, as rescue allows, may also be of benefit.44 If continuous CPR cannot be delivered, a patient with hypothermic cardiac arrest and a core temperature <28◦C (or unknown),should receive 5 min of CPR, alternating with periods ≤5 min without CPR. Patients with a core temperature<20◦C, should receive 5 min of CPR, alternating with periods ≤10min without CPR.45
In the prehospital setting, resuscitation should be withheld in hypothermic patients only if the cause of cardiac arrest is clearly attributable to a lethal injury, fatal illness, prolonged asphyxia, or if the chest is incompressible.46 In all other hypothermic patients, the traditional guiding principle that ‘no one is dead until warm and dead’ should be considered. In remote areas, the impracticalities of achieving rewarming have to be considered.In the hospital setting involve senior doctors and use clinical judgement to determine when to stop resuscitating a hypothermic victim in cardiac arrest.
Modifications to cardio pulmonary resuscitation
- Do not delay careful tracheal intubation when it is indicated. The advantages of adequate oxygenation and protection from aspiration outweigh the minimal risk of triggering VF by performing tracheal intubation.47
- Check for signs of life for up to 1 min. Palpateacentral artery and assess the cardiac rhythm (if ECG monitor available). Echocardiography, near-infrared spectroscopy or ultrasound with Doppler may be used to establish whether there is (an adequate) cardiac output or peripheral blood flow.48, 49 If there is any doubt, start CPRimmediately.
- Hypothermia can cause stiffness of the chest wall, making ventilations and chest compressions difficult. Consider the use of mechanical chest compression devices.50
- Once CPR is underway, confirm hypothermia with a low-reading thermometer.
- The hypothermic heart may be unresponsive to cardio active drugs, attempted electrical pacing and defibrillation. Drug metabolism is slowed, leading to potentially toxic plasma concentrations of any drug given.51 The evidence for the efficacy of drugs in severe hypothermia is limited and based mainly on animal studies. For instance, in severe hypothermic cardiac arrest, the efficacy of amiodarone is reduced.52 Adrenaline may be effective in increasing coronary perfusion pressure, but not survival.53, 54 Vasopressors may also increase the chances of successful defibrillation, but with a core temperature <30◦C, sinus rhythm often degrades back into VF. Given that defibrillation and adrenaline may induce myocardial injury, it is reasonable to withhold adrenaline, other CPR drugs and shocks until the patient has been warmed to a core temperature≥30◦C.Once 30◦C has been reached, the intervals between drug doses should be doubled when compared to normothermia (i.e. adrenaline every 6–10 min). As normothermia (≥35◦C) is approached, use standard drug protocols.
Treatment of arrhythmias. A score temperature decreases, sinus bradycardia tends to give way to atrial fibrillation followed by VF and finally asystole.55, 56 Arrhythmias other than VF tend to revert spontaneously as core temperature increases, and usually do not require immediate treatment.Bradycardia is physiological in severe hypothermia. Cardiac pacing is not indicated unless bradycardia associated with haemodynamic compromise persists after rewarming. The temperature at which defibrillation should firstly be attempted, and how often it should be attempted in the severely hypothermic patient, has not been established. If VF is detected, defibrillate according to standard protocols. If VF persists after three shocks, delay further attempts until core temperature is ≥30◦C.57
CPR and rewarming may have to be continued for several hours to facilitate successfuldefibrillation.
Insulation. General measures for all victims include removal from the cold environment, prevention of further heatloss and rapid transfer to hospital.58 In the field, a patient with moderate or severe hypothermia (hypothermia ≥ II) should be immobilised and handled carefully, oxygenated adequately, monitored(includingECG and core temperature), and the whole body dried and insulated.51
Remove wet clothes while minimising excessive movement of the victim. Removal of wet clothing or use of a vapour barrier seems to be equally effective to limit heat loss.59 Conscious victims (hypothermiaI) can mobilise as exercise rewarms a person more rapidly than shivering.60 Patients will continue cooling after removal from a cold environment (i.e. afterdrop), which may result in a lifethreatening decrease in core temperature triggering a cardiac arrest during transport (i.e.‘rescue death’).Prehospitally, avoid prolonged investigations and treatment, as further heat loss is difficult to prevent. Patients who stop shivering (e.g. hypothermiaII–IV, and sedated or anaesthetised patients) will cool faster.
Prehospital rewarming. Rewarming may be passive, active external, or active internal.InhypothermiaIpassiverewarmingisappropriateaspatientsarestillableto shiver. Passive rewarming is best achieved by full body insulation with wool blankets, aluminium foil, cap and awarmenvironment.In hypothermia II–IV the application of chemical heatpacks to the trunk has been recommended. Inconscious patients who are able to shiver, this improves thermal comfort but does not speed rewarming.61 If the patient is unconscious and the airway is not secured, arrange the insulation around the patient lying in a recovery (lateral decubitus) position. Rewarming in the field with heated intravenous fluids and warm humidified gases is not feasible.51 Intensive active rewarming must not delay transport to a hospital where advanced rewarming techniques, continuous monitoring and observation are available.
Transport. Transport patients with hypothermia stage I to the nearest hospital. In hypothermia stage II–IV, signs of prehospital cardiac instability(i.e. systolic blood pressure <90 mmHg, ventricular arrhythmia, core temperature <28◦C) should determine the choice of admitting hospital. If any signs of cardiac instability are present, transport the patient to an ECLS centre, contacting them well in advance to ensure that the hospital can accept the patient for extracorporeal rewarming.
In hypothermia V, reasons for withholding or terminating CPR should be investigated (e.g.obvious signs of irreversible death, valid DNAR, conditions unsafe for rescuer, avalancheburial ≥ 60 min and airway packed with snow and asystole). In the absence of any of these signs, start CPR and transfer the patient to an ECLS centre. In-hospital rewarming. Unless the patient goes into VF, rewarm using active external methods (i.e. with forced war mair)and minimally invasively methods(i.e. with warm IV infusions). With a core temperature <32◦C and potassium <8m molL− 1, consider LS rewarming.33
Most ECLS rewarmings have been performed using cardiopulmonary bypass, but more recently, veno-arterial extracorporeal membrane oxygenation (VA-ECMO) has become the preferred method due to its rapid availability, the need for less anticoagulation, and the potential to prolong cardio respiratory support after rewarming. I fan ECLS centre is not available, rewarming may be attempted in hospital using adedicated team and a combination of external and internal rewarming techniques (e.g. forced warm air, warm infusions, forced peritoneal lavage).62
Continuous haemodynamic monitoring and warm IV fluids are essential. Patients will require large volumes of fluids during rewarming, as vasodilation causes expansion of the intravascular space. Avoid hyperthermia during and after rewarming.OnceROSC has been achieved use standard post-resuscitation care.
Hyperthermia
Introduction. Hyperthermia occurs when the body’s ability to thermoregulate fails and core temperature exceeds that normally maintained by homeostatic mechanisms. Hyperthermia may be exogenous, caused by environmental conditions, or secondary to endogenous heat production.
Environment-related hyperthermia occurs where heat, usually in the form of radiant energy, is absorbed by the body at a rate faster than can be lost by thermoregulatory mechanisms. Hyperthermia is a continuum of heat-related conditions, starting with heatstress, progressing to heat exhaustion, then to heat stroke and finally to multiple organ dysfunction and cardiac arrest.63
Malignant hyperthermia is a rare disorder of skeletal muscle calcium homeostasis characterised by muscle contracture and life-threatening hypermetaboliccrisisfollowingexposureofgeneticallypredisposedindividualsto halogenated anaesthetics and depolarising muscle relaxants.64, 65
Heatexhaustion
Definition. Heat exhaustion is a non-life-threatening clinical syndrome of weakness, malaise, nausea, syncope, and other nonspecific symptoms caused by heat exposure. Thermoregulation is not impaired. Heat exhaustion is caused by water and electrolyte imbalance due to heat exposure, with or without exertion. Rarely, severe heat exhaustion after physical exertion may be complicated by rhabdomyolysis, myoglobinuria, acute renal failure, and disseminated intravascular coagulation(DIC).
Symptoms. Symptoms are often vague, and patients may not realise that heat is the cause. Symptoms may include weakness, dizziness, headache, nausea, and sometimes vomiting. Syncope due to standing for long periods in the heat (heat syncope) is common and may mimic cardiovascular disorders. On examination, patients appear tired and are usually sweaty and tachy cardic. Mental status is typically normal, unlike in heatstroke. Temperature is usually normal and, when elevated, usually does not exceed 40◦C.
Diagnosis. Diagnosisis clinical and require sex clusion of other possible causes (e.g. hypoglycaemia, acute coronary syndrome, infections). Laboratory testing is require donly if needed to rule out other disorders. Treatment Fluids and electrolyte replacement. Treatment involves removing patients to a cool environment, lying them flat, and giving IV fluids and electrolyte replacement therapy; oral rehydration may not be effective inrapidly replacing electrolytes, but may be amore practical treatment. Rate and volume of rehydration are guided by age, underlying disorders, and clinical response. Replacement of 1–2L crystalloids at 500mLh− 1 is often adequate. External cooling measures are usually not required. Consider external cooling in patients with a core temperature of ≥40◦C.
Heatstroke
Definition. Heat stroke (HS) is defined as hyperthermia accompanied by a systemic inflammatory response with a core temperature>40◦C, accompanied by mental state change and varying levels of organ dysfunction.63
There are two forms of HS:
- Classic (non-exertional) heatstroke (CHS) occurs during high environmental temperaturesandofteneffectstheelderlyduring heat waves.66
- Exertionalheatstroke (EHS)occursduringstrenuousphysical exercise inhigh environmentaltemperaturesand/orhigh humidity and usually effects healthy young adults.67
Mortality from heat stroke ranges between 10 and 50%.68
Predisposing factors. The elderly are at increased risk for heat-related illness because of underlying illness, medication use, declining thermoregulatory mechanisms and limited social support. There are several risk factors: lack of acclimatisation, dehydration, obesity, alcohol, cardiovascular disease, skin conditions (psoriasis, eczema, scleroderma, burn, cystic fibrosis), hyperthyroidism,phaeochromocytomaanddrugs(anticholinergics, diamorphine, cocaine, amphetamine, phenothiazines, sympathomimetics, calcium channel blockers,beta-blockers).
Symptoms. Heat stroke can resemble septic shock and may be caused by similar mechanisms.69 A single centre case series reported 14 ICU deaths in 22 heatstroke patients admitted to ICU with multiple organ failure.70 Features included:
- coretemperature≥40◦C;
- hot, dry skin(sweating present in about 50% of cases of exertional heatstroke);
- early signs and symptoms (e.g. extreme fatigue, headache, fainting, facial flushing, vomiting and diarrhoea);
- cardiovascular dysfunction including arrhythmias and hypotension71;
- respiratory dysfunction including acute respiratory distress syndrome (ARDS)72;
- central nervous system dysfunction including seizures and coma73;
- liver and renal failure74;
- coagulopathy;
- rhabdomyolysis.75
Other clinical conditions presenting with increased core temperature need to be considered, including drug toxicity, drug withdrawal syndrome, serotonin syndrome, neuroleptic malignant syndrome, sepsis, central nervous system infection, endocrine disorders (e.g. thyroidstorm, phaeochromocytoma).
Treatment. The main stay of treatment is supportive therapy and rapidly cooling the patient.77–78 Start cooling in the prehospital setting if possible.Aim to rapidly reduce the core temperature to approximately 39◦C. Patients with severe heatstroke need to be managed in an ICU environment. Large volumes of fluids and correction of electrolyte abnormalities may be required (seehypo/hyperkalaemia and other electrolyte disorders).
Cooling techniques. Several cooling methods have been described, but ther eare few formal trials to determine which is optimal. Simple cooling techniques include drinking cold fluids, fanning the completely undressed patient and spraying tepid water on the patient. Ice packs over areas where there are large superficial blood vessels(axillae, groins, neck) may also be useful. Surface cooling methods may cause shivering. Incooperative stable patients, immersion in cold water can be effective79; however, this may cause peripheral vasoconstriction, shunt blood away from the periphery and reduce heat dissipation.Immersion is also not practical in the sickest patients. Further techniques to cool patientswith hyperthermia are similar to those used for targeted temperature management after cardiac arrest (see post resuscitation care).80
Cold intravenous fluids will decrease body temperature. Gastric, peritoneal,81 pleural or bladder lavage with cold water will lower the core temperature.
Intravascular cooling techniques include the use of cold IV fluids,82 intravascular cooling catheters83, 84 and extracorporeal circuits,85 e.g. continuous veno-venoushaemo filtration or cardiopulmonary bypass.
Pharmacological treatment.There are no specific drug therapies in heat stroke that lower core temperature.There is no good evidence that antipyretics (e.g. nonsteroidal anti-inflammatory drugs or paracetamol) are effective in heat stroke. Diazepam may be useful to treat seizures and facilitate cooling.86 Dantrolene has not been shown to be beneficial.87–89
Malignanthyperthermia
Malignant hyperthermia is a life-threatening genetic sensitivity of skeletal muscles to halogenated volatile anaesthetics and depolarising neuromuscular blocking drugs, occurring during or after anaesthesia.90 Stop triggering agents immediately; give oxygen, correct acidosis and electrolyte abnormalities. Start active cooling and give dantrolene.91 Other drugs such as 3,4-methylenedioxy methamphetamine (MDMA, ‘ecstasy’) and amphetamines also cause a condition similar to malignant hyperthermia and the use of dantrolene may be beneficial.92 Modifications to cardio pulmonary resuscitation. There are no specific studies of cardiac arrest in hyperthermia. If cardiac arrest occurs, follow standard guidelines and continue cooling the patient. Use the same cooling techniques as for targeted temperature management after cardiac arrest (see Section 5 Post-resuscitation care).80 Attempt defibrillation using standard energy levels. Animal studies suggest the prognosis is poor compared with normothermic cardiac arrest.93, 94 The risk of unfavourable neurological outcome increases by 2.26 (oddsratio) for each degree of body temperature >37◦C.
Hypovolaemia
Introduction
Hypovolaemia is a potentially treatable cause of cardiac arrest that usually results from a reduced intravascular volume (i.e. haemorrhage), but relative hypovolaemia may also occur in patients with severe vasodilation (e.g.anaphylaxis, sepsis). Hypovolaemia from mediator-activated vasodilation and increased capillary permeability is a major factor causing cardiac arrest in severe anaphylaxis. 96
Hypovolaemia from blood loss, is a leading cause of death in traumatic cardiac arrest. 97 External blood loss is usually obvious, e.g. trauma, haematemesis, haemoptysis, but may be more challenging to diagnose when occult, e.g. gastrointestinal bleeding or rupture of an aortic aneurysm. Patients undergoing major surgery are at high-risk from hypovolaemia due to post-operative haemorrhage and must be appropriately monitored (see perioperative cardiac arrest).
Depending on the suspected cause, initiate volume therapy with warmed blood products and/or crystal loids, in order to rapidly restore intravascular volume. At the same time, initiate immediate intervention to control haemorrhage, e.g. surgery, endoscopy, endovascular techniques,98 or treat the primary cause (e.g. anaphylactic shock).In the initial stages of resuscitation use any crystal loid solution that is immediately available. If there is a qualified sonographer able to perform ultrasound without interruption to chest compressions, e.g. during rhythm check or ventilations, it may be considered as an additional diagnostic too linhypovolaemic cardiac arrest.
Treatment recommendations for cardiac arrest and periarrest situations in anaphylaxis and trauma are addressed in separate sections because of the need for specific therapeutic approaches.
Anaphylaxis
Definition. A precise definition of anaphylaxis is not important for its emergency treatment.99 The European Academy of Allergy and Clinical Immunology Nomenclature Committee proposed the following broad definition:100 anaphylaxis is a severe, life threatening, generalised or systemic hypersensitivity reaction. This is characterised by rapidly developing life-threatening airway and/or breathing and/or circulation problems usually associated with skin and mucosal changes.1, 96, 101, 102
Epidemiology. Anaphylaxis is common and affects about 1 in 300 of the European population at some stage in their lives, with an incidence from 1.5 to 7.9 per 100,000 person-years.103
Anaphylaxis can be triggered by any of a very broad range of triggers with food, drugs, stinging insects, and latex the most commonly identified triggers.103 Food is the commonest trigger in children and drugs the commonest in adults.104
Virtually any food or drug can be implicated, but certain foods (nuts) and drugs (muscle relaxants, antibiotics, non steroidal anti-inflammatory drugs and aspirin) cause most reactions.104
A significant number of cases of anaphylaxis are idiopathic. Between 1992 and 2012 in the UK, admission and fatality rates for drug- and insect sting-induced anaphylaxis were highest in the group aged 60 years and older. In contrast, admissions due to food-triggered anaphylaxis were most common in young people, with a marked peak in the incidence offatalfood reactions during the second and third decades of life.106
The overall prognosis of anaphylaxis is good, with a case fatality ratio of less than 1% reported in most population-based studies. The European Anaphylaxis Registry reported that only 2% of 3333 cases were associated with cardiac arrest.107 If intensive care unit admission is required, survival to discharge is over 90%. Over the period 2005– 2009, there were 81 paediatric and 1269 adult admissions with anaphylaxis admitted to UK critical care units. Survival to discharge was 95% for children, and 92% for adults.108
Anaphylaxis and risk of death is increased in those with preexisting asthma, particularly if the asthmais poorly controlled, severe or in asthmatics who delay treatment.109, 110 When anaphy laxisisfatal, death usually occurs very soon after contact with the trigger. From a case series, fatal food reactions cause respiratory arrest typically within 30–35 min; insect stings cause collapse from shock within 10–15 min; and deaths caused by intravenous medication occur most commonly within 5 min. Death never occurred more than 6h after contact with the trigger.101, 111
Recognition of ananaphylaxis. Anaphylaxis is the likely diagnosis if a patient who is exposed to a trigger (allergen) develops a sudden illness(usually within minutes) with rapidly developing life-threatening airway and/or breathing and/or circulation problems usually associated with skin and mucosal changes. The reactionis usually unexpected.
The European Academy of Allergy and Clinical Immunology’s (EAACI) Taskforce on Anaphylaxis state that anaphylaxis is highly likely when any one of the following three criteria is fulfilled96, 112:
- Acute on set of an illness (minutes to several hours) with involvement of the skin, mucosal tissue, or both (e.g. generalised hives, pruritus or flushing, swollen lips– tongue–uvula) and at least one of the following:
- a. Respiratory compromise, e.g. dyspnoea, wheeze– bronchospasm, stridor, reduced peak expiratory flow(PEF), hypoxaemia.
- b. Reduced blood pressure or associated symptoms of end-organ dysfunction, e.g.hypotonia(collapse), syncope, incontinence.
- Two or more of the following that occur rapidly after exposure to a likely allergen for that patient(minutes to several hours):
- a. Involvement of the skin–mucosal tissue, e.g. generalised hives, itch-flush, swollen lips–tongue–uvula.
- b. Respiratory compromise, e.g. dyspnoea, wheeze– bronchospasm, stridor, reduced PEF, hypoxaemia.
- c. Reduced blood pressure or associated symptoms, e.g. hypotonia(collapse), syncope, incontinence.
- d. Persistent gastrointestinal symptoms, e.g. crampy abdominal pain, vomiting.
- Reduced blood pressure after exposure to known allergen for that patient (minutes to several hours):
- a. Infants and children: low systolic blood pressure(<70mmHg from 1 month to 1 year;<70mmHg+(2×age) from 1 year to 10years;<90mm Hg from 11 to 17 years)or>30% decrease in systolic blood pressure.
- b. Adults: systolic blood pressure of <90 mmHg or >30% decrease from that person’s baseline.
Treatment. The evidence supporting specific interventions for the treatment of anaphylaxis is limited.113 A systematic ABCDE approach to recognise and treat anaphylaxis is recommended with immediate administration of intramuscular(IM) adrenaline (Fig. 4.2).Treat life-threatening problems as you find them. The basic principles of treatment are the same for all age groups. Monitor all patients who have suspected anaphylaxis as soon as possible (e.g. by ambulance crew, in the emergency department, etc.). Minimum monitoring includes pulseoximetry, non-invasive blood pressure and a 3-lead ECG.
Patient positioning. Patients with anaphylaxis can deteriorate and are at risk of cardiac arrest if made to sit up or stand up.114 All patients should be placed in a comfortable position. Patients with airway and breathing problems may prefer to sit up, as this will make breathing easier. Lying flat with or without leg elevation is helpful for patients with a low blood pressure.
Remove the trigger (if possible). Stop any drug suspected of causing anaphylaxis. Remove the stinger after a bee/wasp sting. Early removal is more important than the method of removal.115 Do not delay definitive treatment if removing the trigger is not feasible.
Cardiac arrest following anaphylaxis. Start CPR immediately and follow current guidelines. Prolonged CPR may be necessary. Rescuers should ensure that help is on its way as early ALS is essential.
Airway obstruction.Anaphylaxis can cause airway swelling and obstruction. This will make airway and ventilation interventions (e.g. bag-mask ventilation, tracheal intubation, cricothyroidotomy) difficult. Consider early tracheal intubation before airway swelling makes this difficult. Call for expert help early.
Adrenaline (first line treatment). Adrenaline is the most important drug for the treatment of anaphylaxis.116, 117 Although there are no randomised controlled trials,118 adrenaline is a logical treatment and there is consistent anecdotal evidence supporting its use to ease bronchospasm and circulatory collapse. As an alpha-receptor agonist, it reverses peripheral vasodilation and reduces oedema. Its beta-receptor activity dilates the bronchial airways, increases the force of myocardial contraction, and suppresses histamine and leukotriene release. Activation of beta-2 adrenergic receptors on mast cell surfaces inhibit their activation, and early adrenaline attenuates the severity of IgE-mediated allergic reactions. Adrenaline is most effective when given early after the onset of the reaction,119 and adverse effects are extremely rare with correct IM doses. Give adrenaline to all patients with life-threatening features. If these features are absent but there are other features of a systemic allergic reaction, the patient needs careful observation and symptomatic treatment using the ABCDE approach. Intramuscular adrenaline. The intramuscular (IM) route is the best for most individuals who have to give adrenaline to treat anaphylaxis. Monitor the patient as soon as possible (pulse, blood pressure, ECG, pulseoximetry). This will help monitor the response to adrenaline. The IM route has several benefits:
- There is a greater margin of safety.
- It does not require intravenous access.
- The IM route is easier to learn.
- Patients with known allergies can self-administer IM adrenaline.
The best site for IM injection is the anterolateral aspect of the middle third of the thigh. The needle for injection needs to belong enough to ensure that the adrenaline is injected into muscle.120
The subcutaneous or inhaled routes for adrenaline are not recommended for the treatment of anaphylaxis because they are less effective than the IM route.121–123
Adrenaline intramuscular dose. The evidence for the recommended doses is limited. The EAACI suggests IM adrenaline (1mg mL− 1 ) should be given a dose of 10 mcg kg− 1 of body weight to a maximum total dose of 0.5mg.96
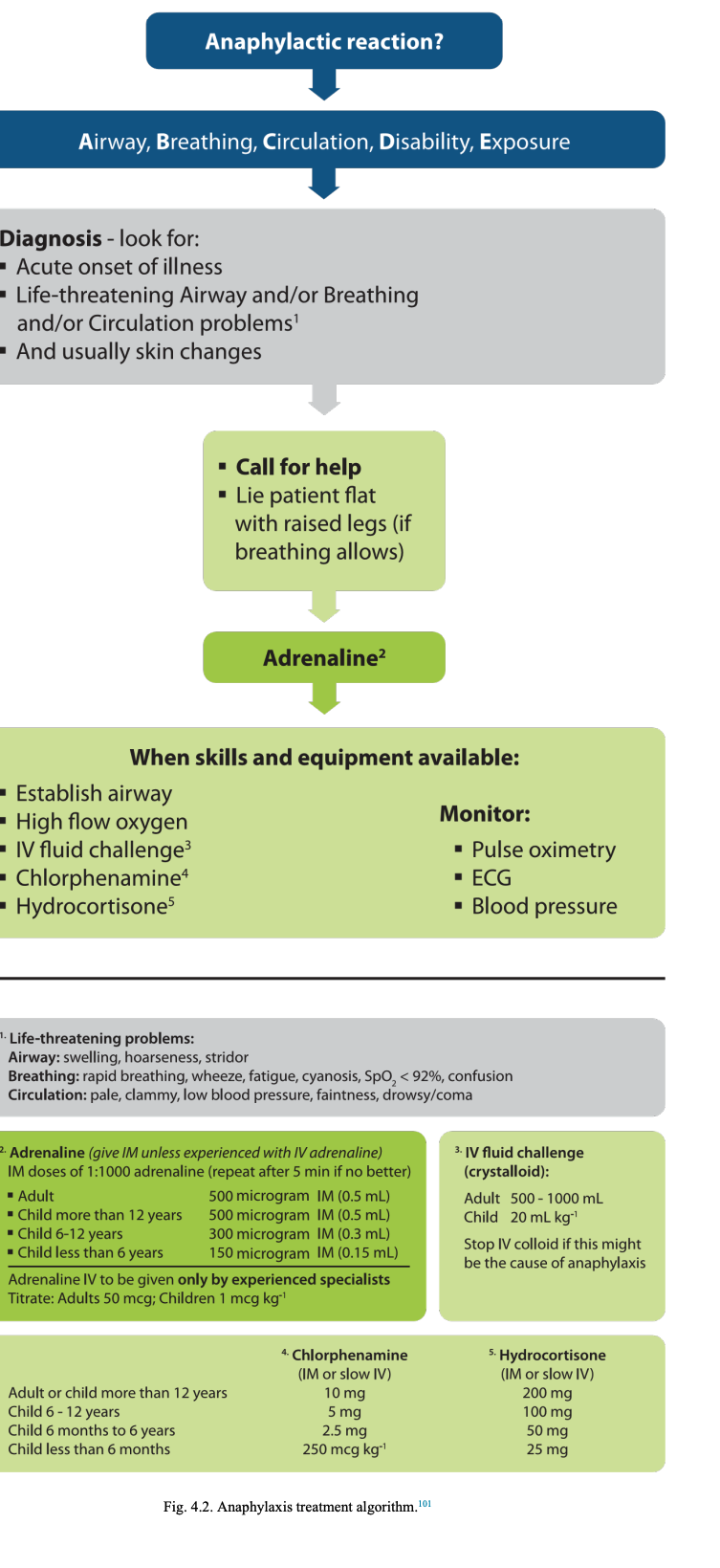
The following doses are based on what is considered to be safe and practical to draw up and inject in an emergency (equivalent volume of 1:1000 adrenaline is shown in brackets):
12 years and adults 500 microgram IM (0.5mL)
6–12 years 300 microgram IM (0.3 mL)
6 months–6years 150 microgram IM (0.15 mL)
<6months 150 microgram IM (0.15 mL)
Repeat the IM adrenaline dose if there is no improvement in the patient’s condition within 5 min. Further doses can be given at about 5-min intervals according to the patient’s response.
Intravenous adrenaline (for specialist use only). There is a much greater risk of causing harmful side effects by inappropriate dosage or misdiagnosis of anaphylaxis when using intravenous (IV) adrenaline.124 IV adrenaline should only be used by those experienced in the use and titration of vasopressors in their normal clinical practice(e.g. anaesthetists, emergency physicians, intensive care doctors). In patients with a spontaneous circulation, IV adrenaline can cause life-threatening hypertension, tachycardia, arrhythmias, and myocardial is chaemia. If IV access is not available or not achieved rapidly, use the IM route for adrenaline. Patients who are given IV adrenaline must be monitored – continuous ECG and pulse oximetry and frequent non-invasive blood pressure measurements as a minimum. Patients who require repeated IM doses of adrenaline may benefit from IV adrenaline. It is essential that these patients receive expert help early.
Adrenaline intravenous dose(for specialist use only).
- Adults: Titrate IV adrenaline using 50 microgram bol uses according to response. If repeated adrenaline doses are needed, start an IV adrenaline infusion.125, 126
- Children: IM adrenaline is the preferred route for children having an aphylaxis. The IV route is recommended only in specialist paediatric settings by those familiar with its use (e.g. paediatric anaesthetists, paediatric emergency physicians, paediatric intensivists) and if the patient is monitored and IV access is already available. There is no evidence on which to base a dose recommendation– the dose is titrated according to response. A child may respond to a dose as small as 1 mcg kg− 1. This requires very careful dilution and checking to prevent dose errors.
Adrenaline intravenous/intraosseous dose(incardiac arrest only). Cardiac arrest with suspected anaphylaxis should be treated with standard doses of IV or intraosseous (IO) adrenaline for cardiac arrest. If this is not feasible, consider IM adrenaline if cardiac arrest is imminentor has just occurred.
Oxygen (give as soon as available). Initially, give the highest concentration of oxygen possible using a mask with anoxygen reservoir.127 Ensure high-flow oxygen (usually greater than 10 L min− 1 to prevent collapse of the reservoir during inspiration.
If the patient’s trachea is intubated, ventilate the lungs with high concentration oxygen using a self-inflating bag.
Fluids (give as soon as available). Large volumes of fluid may leak from the patient’s circulation during anaphylaxis. There will also be vasodilation. If IV access has been gained, infuse IV fluids immediately. Give a rapid IV fluid challenge (20 mL kg− 1) in a child or 500–1000 mL in an adult and monitor the response; give further doses as necessary. There is no evidence to support the use of colloids over crystalloids in this setting. Consider colloid infusion as a cause in a patient receiving a colloid at the time of onset of an anaphylaxis and stop the infusion. A large volume of fluid maybe needed.
If IV access is delayed or impossible, the IO route can be used for fluids or drugs.Do not delay the administration of IM adrenaline while attempting IO access.
Antihistamines(give after initial resuscitation). Antihistamines area second line treatment for anaphylaxis.The evidence to support their use is limited, butthere are logical reasons for their use.128 H -antihistamines help counter histamine-mediated 1 vasodilation, broncho constriction, and particularly cutaneous symptoms. There is little evidence to support the routine use of an H2-antihistamine (e.g. ranitidine, cimetidine) for the initial treatment of anaphylaxis.
Glucocorticosteroids(give after initial resuscitation).Corticosteroids may help prevent or shorten protracted reactions, although the evidence is limited.129 In asthma, early corticosteroid treatment is beneficial in adults and children. There is little evidence on which to base the optimum dose of hydrocortisone in anaphylaxis.
Other drugs.
Bronchodilators. The presenting symptoms and signs of severe anaphylaxis and life-threatening asthma can be the same. Consider further bronchodilator therapy with salbutamol (inhaled or IV), ipratropium (inhaled), aminophylline (IV) or magnesium (IV) (see asthma). IV magnesium is a vasodilator and can make hypotension worse.
Cardiacdrugs. Adrenaline remains the first linevasopressor for thetreatment of anaphylaxis. There are animal studies and case reports describing the use of other vasopressors and inotropes (noradrenaline, vasopressin, terlipressin metaraminol, methoxamine, and glucagon) when initial resuscitation with adrenaline and fluids has not been successful.130–142 Use these drugs only in specialist settings (e.g.ICU) where there is experience in their use. Glucagon can be useful to treat anaphylaxis in a patient taking a beta-blocker.143
Some case reports of cardiac arrest suggest cardiopulmonary bypass144, 145 or mechanical chest compression devices may also be helpful.146
Investigations. Undertake the usual investigations appropriate for a medical emergency, e.g. 12-lead ECG, chest X-ray, urea and electrolytes, arterial blood gases, etc.
Mast cell tryptase. The specific test to help confirm a diagnosis of anaphylaxisis measurement of mast cell tryptase. Tryptase is the major protein component of mast cell secretory granules. In anaphylaxis, mast cell degranulation leads to markedly increased blood tryptase concentrations. Tryptase concentrations in the blood may not increase significantly until 30 min or more after the onset of symptoms, and peak 1–2h after onset.147 The half-life of tryptase is short (approximately2h), and concentrations may be back to normal within 6–8h, so timing of any blood samples is very important. The time of onset of the anaphylaxis is the time when symptoms were first noticed. (a) Minimum: one sample at 1–2 h after the start of symptoms. (b) Ideally: Three timed samples:
- Initial sample as soon as feasible after resuscitation has started – do not delay resuscitation to take sample.
- Second sample at 1–2h after the start of symptoms.
- Third sample either at 24h orinconvalescence (for example in a follow-up allergy clinic).This provides baseline tryptase levels– some individuals have an elevated baseline level.
Serial samples have better specificity and sensitivity than a single measurement in the confirmation of anaphylaxis.148 Discharge and follow-up. Patients who have had suspected anaphylaxis (i.e. an airway, breathing or circulation problem)should be treated and then observed in a clinical area with facilities for treating life-threatening ABC problems. Patients with a good response to initial treatment should be warned of the possibility of an early recurrence of symptoms and in some circumstances should be kept underobservation. The exact incidence of biphasic reactions is unknown. Although studies quote an incidence of 1–20%, it is not clear whether all the patients in these studies actually had anaphylaxis or whether the initial treatment was appropriate.149 There is no reliable way of predicting who will have a biphasic reaction. It is therefore important that decisions about discharge are made for each patient by an experienced clinician.
Before discharge from hospital, all patients must:
- Be reviewed by an allergy specialist and have a treatment plan based on their individual risk.
- Be given clear instructions to return to hospital if symptoms return.
- Be considered for an adrenaline auto-injector, or given a replacement150–152 and ensured that appropriate training has beengiven.
- Have a plan for follow-up, including contact with the patient’s general practitioner.
Patients need to know the allergen responsible (if identified) and how to avoid it. Patients need to be able to recognise the early symptoms of anaphylaxis, so that they can summon help quickly and prepare to use the ir emergency medication.Although there are no randomised clinical trials, there is evidence that individualised action plans for self-management should decrease the risk of recurrence.153
Traumatic cardiac arrest
Introduction. Traumatic cardiac arrest (TCA) carries a very high mortality, but in those where ROSC can be achieved, neurological outcome in survivors appears to be much better than in other causes of cardiac arrest.154, 155 The response to TCA is time critical and success depends on a well-established chain of survival, including advanced prehospital and specialised trauma centre care. Immediate resuscitative efforts in TCA focus on simultaneous treatment of reversible causes, which takes priority over chest compressions.
Diagnosis. The diagnosis of traumatic cardiac arrest is made clinically. The patient presents with agonal or absent spontaneous respiration and absence of a central pulse.
A peri-arrest state is characterised by cardiovascular instability, hypotension, loss of peripheral pulses in uninjured regions and adeteriorating conscious level without obvious central nervous system (CNS) cause. If untreated, this state is likely to progress to cardiac arrest. Rapid focused ultrasound assessment may be helpful in the immediate diagnosis and management, but should not delay resuscitative interventions.156
It is vitalthat a medical cardiac arrestis not misdiagnosed as a TCA and must be treated with the universal ALS algorithm. Cardiac arrest or other causes of sudden loss of consciousness (e.g. hypoglycaemia, stroke, seizures) may cause a secondary traumatic event. Some observational studies have reported that about 2.5% of non traumatic OHCAs occur in cars.157–159 In these cases, shockable rhythms (VF/pVT) are more common.97
The primary cause of the cardiac arrest can be elucidated from information about past medical history, events preceding the accident (if possible), and a systematic post-ROSC assessment, including a 12-lead ECG.
Prognostic factors and with holding resuscitation.There are no reliable predictors of survival for traumatic cardiac arrest. Factors that are associated with survival include the presence of reactive pupils, an organised ECG rhythm and respiratory activity.159, 160
Short duration of CPR and prehospital times have also been associated with positive outcomes.161
A large systematic review reported an overall survival rate of 3.3% in blunt and 3.7% in penetrating trauma, with good neurological outcome in 1.6% of all cases.154 Outcome is age dependent, with children having a better prognosis than adults.97, 154
There is considerable variation in reported mortality (range 0–27%) reflecting heterogeneity in case mix and care in different systems. PEA, which in TCA may initially be a low output state, and a systole are the prevalent heart rhythms in TCA. Ventricular fibrillation(VF)is rare but carries the best prognosis.97, 155
One study reported good neurological outcome in 36.4% of TCA patients presenting with VF, but only in 7% with PEA and 2.7% of those in a systole,155 but other studies of patients in non-shockable rhythms have reported 100%mortality.159, 162, 163
The American College of Surgeons and the National Association of EMS physicians recommend withholding resuscitation in situations where death is inevitable or established and in trauma patients presenting with apnoea, pulselessness and without organised ECG activity.164
However, neurologically intact survivors initially presenting in this state have been reported.155 We therefore recommend the following approach: Consider with holding resuscitation in TCA in any of the following conditions:
- no signs of life within the preceeding 15 min;
- massive trauma incompatible with survival (e.g. decapitation, penetrating heart injury, loss of brain tissue).
We suggest termination of resuscitative efforts should be considered if there is:
- no ROSC after reversible causes have been addressed;
- no detectable ultra sonographic cardiac activity.
Trauma caresystems throughout Europe vary considerably and we recommend establishing regional guidelines for treatment of TCA and tailoring patient pathways to infrastructure and resources.
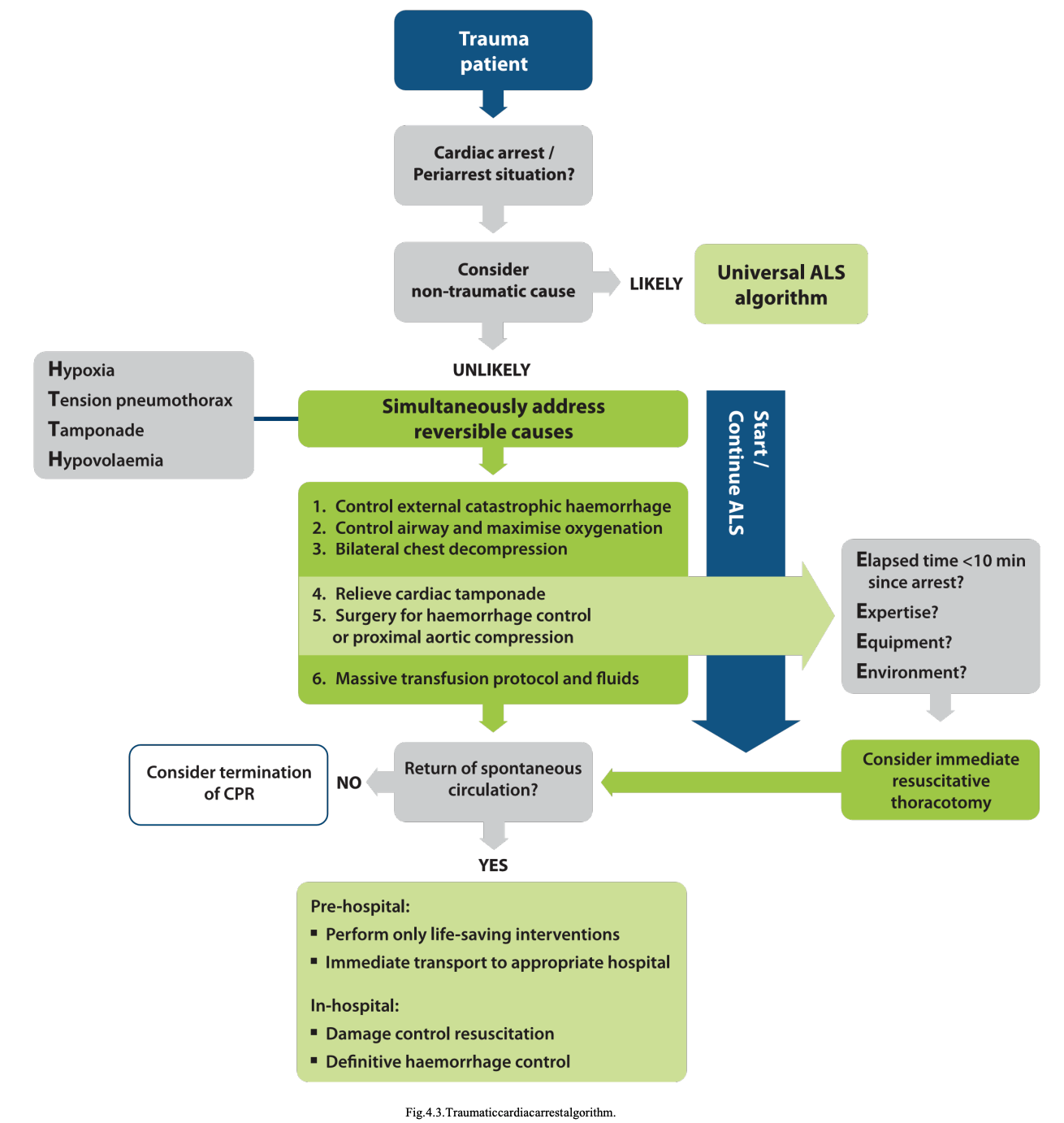
Treatment. Emphasis on rapid treatment of all potentially reversible pathology is the basis of treatment guidelines. These principles are addressed in several treatment algorithms.97, 165–167
All algorithms attempt to rapidly address reversible causes of TCA in the prehospital and in-hospital phases of care. Fig. 4.3 shows a traumatic cardiac (peri-) arrest algorithm, which is based on the universal ALS algorithm.168
Effectiveness of chest compressions. Chest compressions are still the standard of care in patients with cardiac arrest, irrespective of aetiology. In cardiac arrest caused by hypovolaemia, cardiactamponadeor tensionpneumothorax, chest compressions are unlikely to be as effective as in normovolaemic cardiac arrest.169–172
Because of this fact, chest compressions take a lower priority than the immediate treatment of reversible causes, e.g. thoracotomy, controlling haemorrhage, etc. In an out-of-hospital setting, only essential life-saving interventions should be performed on scene followed by rapid transfer to the nearest appropriate hospital.
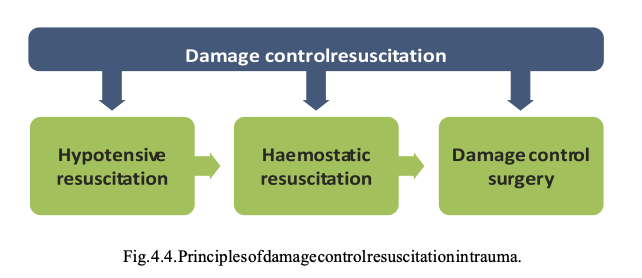
Hypovolaemia. Uncontrolled haemorrhage is the cause of traumatic cardiac arrest in 48% of all TCA.97 The treatment of severe hypovolaemic shock has several elements. The main principle is to achieve ‘haemostasis without delay’, usually with surgical or radiological intervention. Temporaryhaemorrhagecontrolcanbe lifesaving:
- Treat compressible external haemorrhage with direct pressure (with or without a dressing), use tourniquets if needed and/or apply topical haemostatic agents.173
- Non-compressible haemorrhage is more difficult. Use splints (pelvic splint), blood products, intravenous fluids and tranexamic acid while moving the patient to surgical haemorrhage control.
Over the past 10 years the principle of ‘damage control resuscitation’ has been adopted in trauma resuscitation for uncontrolled haemorrhage. Damage control resuscitation combines permissive hypotension and haemostatic resuscitation with damage control surgery. Limited evidence174 and general consensus have supported a conservative approach to intravenous fluid infusion, with permissive hypotension untilsurgical haemostasisis achieved. Permissive hypotension allows intravenous fluid administration to a volume sufficient to maintain a radial pulse.175, 176
Haemostatic resuscitation is the very early use of blood products as primary resuscitation fluid to prevent exsanguination by trauma-induced coagulopathy.177 The recommended ratio of packed red cells, fresh frozen plasma and platelets is 1:1:1.178 Some services have also started using blood products in the prehospital phase of care.179, 180 Simultaneous damage control surgery and haemostatic resuscitation using massive transfusion protocols (MTP)173 are the principles of damage control resuscitation in patients with exsanguinating injuries (Fig. 4.4).177
Although the evidence for permissive hypotension during resuscitation is limited, particularly with regards to blunt trauma, permissive hypotension has been endorsed in both civilian and military care,181 generally aiming for a systolic blood pressure of 80–90mm Hg. Caution is advised with this strategy in patients with traumatic brain injury were a raised intracranial pressure may require a higher cerebral perfusion pressure. The duration of hypotensive resuscitation should not exceed 60 min, because the risks of irreversible organ damage then exceed its intended benefits.176 Tranexamicacid (TXA)(loading dose 1g over 10 min followed by infusion of 1g over 8 h) increases survival from traumatic haemorrhage.182 It is most effective when administered within the first hour and certainly within the first 3h following trauma.182
Give TXA in the prehospital setting when possible. Hypoxaemia. Hypoxaemia due to airway obstruction and traumatic as phyxiah as been reported as cause of traumatic cardiac arrest in 13% of all cases.97 Effective airway management and ventilation can reverse hypoxic cardiac arrest and it is essential to establish and maintain oxygenation of trauma patients with a severely compromised airway. Tracheal intubation in trauma patients is a difficult procedure with a high failure rate if carried out by less experienced care providers.183, 184 Use basic airway manoeuvres and second-generation supraglottic airways to maintain oxygenation if tracheal intubationcannotbeaccomplished immediately. Positive pressure ventilation worsens hypotension by impeding venous return to the heart, particularly in hypovolaemic patients.185 Low tidal volumes and slow respiratory rates may help optimise cardiac preload. Monitor ventilation with continuous waveform capnography and adjust to achieve normocapnia.173
Tension pneumothorax. Thirteen percent of all cases of TCA are caused by tension pneumothorax.97 To decompress the chest in TCA, perform bilateral thoracostomies in the 4th intercostal space, extending to a clamshell thoracotomy if required.In the presence of positive pressure ventilation, thoracostomies are likely to be more effective than need lethoracocentes is and quicker than inserting a chest tube (see tension pneumothorax).186, 187
Cardiac tamponade and resuscitative thoracotomy. Cardiac tamponade is the underlying cause of approximately 10% of cardiac arrest in trauma.97 Where there is TCA and penetrating trauma to the chest or epigastrium, immediate resuscitative thoracotomy (RT) via a clamshell incision188 can be life saving.189 The chance of survival is about 4 times higher in cardiac stab wounds than in gunshot wounds.190 Resuscitative thoracotomy is also applied for other life threatening injuries; the evidence was examined in 2012191 and guidelines produced which recommend that, after arrival in hospital, the decision to proceed with RT should include the following criteria:
- blunt trauma patients with less than 10 min of prehospital CPR;
- penetrating torso trauma patients with less than 15 min of CPR. These guidelines estimate survival rates for RT of approximately 15% for all patients with penetrating wounds and 35% for patients with a penetrating cardiac
These guidelines estimate survival rates for RT of approximately 15% for all patients with penetrating wounds and 35% for patients with a penetrating cardiac wound. In contrast, survival from RT following blunt trauma is dismal, with survival rates of 0–2% being reported.191, 192 Successful RT is time critical. One UK service recommends that if surgical interventionv cannot be accomplished within 10 min after loss of pulse in patients with penetrating chest injury, on scene RT should be considered.10 Based on this approach, of 71 patients who underwent RT at scene, 13 patients survived and 11 of these made a good neurological recovery. The prerequisites for a successful RT can be summarised as the ‘four Es rule’(4E):
- Expertise: teams that perform RT must be led by a highly trained and competent healthcare practitioner. These teams must operate under a robust governance framework.
- Equipment: adequate equipment to carry out RT and to deal with the intrathoracic findings is mandatory.
- Environment: ideally RT should be carried out in an operating theatre. RT should not be carried out if there is inadequate physical access to the patient, or if the receiving hospital is not easy to reach.
- Elapsed time: the time fromloss of vital signs to commencing a RT should not be longer than 10min. If any of the four criteria is not met, RT is futile and exposes the team to unnecessary risks.193
If any of the four criteria is not met, RT is futile and exposes the team to unnecessary risks.193
Needle aspiration of tamponade, with or without ultrasound guidance, is unreliable because the pericardium is commonly full of clotted blood.194, 195 If thoracotomy is not possible, however, consider ultrasound guided pericardiocentes is to treat TCA associated with suspected cardiac tamponade. Non-image guided pericardiocentes is is an alternative,only if ultrasound is not available.Placement of a pericardialdrain may be beneficial in some patients. Diagnostics. Ultrasonography should be used in the evaluation of the compromised trauma patient to target life-saving interventions if the cause of shock cannot be established clinically.196, 173
Haemoperitoneum, haemo- or pneumothorax and cardiac tamponade can be diagnosed reliably in minutes, even in the prehospital phase.197 Early whole-body computed tomography (WBCT) scanning as part of the primary survey may improve outcome in major trauma.198 WBCT is increasingly employed to identify the source of shock and to guide subsequent haemorrhage control. Prehospital care. Short prehospital times are associated with increased survival rates for major trauma and TCA.The time elapsed between injury and surgical controlof bleeding should therefore be minimised and the patient should be immediately transferred to a trauma centre for ongoing damage control resuscitation.173 A ‘scoop and run’ concept for these patients may be lifesaving.
Tensionpneumothorax
Introduction
Tension pneumothorax defined as haemodynamic compromise in a patientwith an expanding in trapleural air mass is a treatable cause of cardiac arrest and should be excluded during CPR.199 Tension pneumothorax can occur in a variety of clinical situations including trauma, asthma and other respiratory disease, but can also be iatrogenic following invasive procedures, e.g. attempts at central venous catheter insertion. It is more common and often more severe in patients undergoing positive pressure ventilation.200 The incidence of tension pneumothorax is approximately 5% in major trauma patients treated in the prehospital setting (13% of those developing TCA), and less than 1% of adults admitted to ICU.97, 201, 202
Diagnosis
Diagnosis of tension pneumothorax in a patient with cardiac arrest or haemodynamic instability must be based on clinical examination. The symptoms include haemodynamic compromise (hypotensionor cardiac arrest) in conjunction with signssuggestive of a pneumothorax (preceding respiratory distress, hypoxia, absent unilateral breath sounds on auscultation, subcutaneous emphysema) and mediastinal shift(tracheal deviation and jugularvenous distention).200 During CPR, presentation is not always classical, but when it is suspected in the presence of cardiac arrest or severe hypotension, chest decompression should be carried out immediately before radiographic confirmation.201
Treatment
Needle decompression. Needle chest decompression is rapid and within the skillset of most ambulance personnel but is of limited value.203, 204 A significant proportion of patients have chest wall thickness which makes needle decompression with a standard length 14-gauge cannula ineffective.205 Cannulae are also prone to kinking and blockage.206 Any attempt at needle decompression should be followed by insertion of a chest tube(see asthma).
Thoracostomy. Tracheal intubation, positive pressure ventilation and formal chest decompression effectively treats tension pneumothorax in patients with TCA. Simple thoracostomy is easy to perform and used routinely by several prehospital physician services.187, 207 This consists of the first stage of standard chest tube insertion–as impleincision and rapid dissection into the pleural space in the positive pressure entilated patient (see traumatic cardiac arrest). Chest tube insertion is then carried out after the resuscitation phase. This requires additional equipment, takes longer to perform and creates a closed system that has the potential tore-tension. Chest drain tubes may become blocked with lung or blood clots and have the potential to kink.
Tamponade
Introduction
Cardiac tamponade occurs when the pericardial sac is filled with fluid under pressure, which leads to compromise of cardiac function and ultimately cardiac arrest. The condition most commonly occurs after penetrating trauma and cardiac surgery. Mortality is high and immediate decompression of the pericardium is required to give any chance of survival.
Treatment Thoracotomy. The criteria and prerequisites for resuscitative thoracotomy in patients with penetrating trauma to the chest or epigastrium are described in section on traumatic cardiac arrest. Treatment of the tamponade following cardiac surgery is addressed in the section on cardiac arrest following cardiac surgery. Pericardiocentes is. If thoracotomy is not possible, consider ultrasound-guided pericardiocentes is to treat cardiac arrest associated with suspected traumatic or non traumatic cardiac tamponade. Non-image guided pericardiocentesis is an alternative, only if ultrasound is not available.
Thrombosis
Pulmonary embolism Introduction. Cardiac arrest from acute pulmonary embolism is the most serious clinical presentation of venous thromboembolism, in most cases originating from a deep venous thrombosis (DVT).208
The reported incidence of cardiac arrest caused by pulmonary embolism is 2–9% of all OHCAs,209–212 and 5–6% of all in-hospital cardiac arrests.213, 214 but it is likely to be underestimated. Overall survival is low.211,215 Specific treatments for cardiac arrest resulting from pulmonary embolism include administration of fibrinolytics, surgical embolectomy and percutaneous mechanical thrombectomy.
Diagnosis. Diagnosis of acute pulmonary embolism during cardiac arrest is difficult. One study has reported correct recognition of the underlying causes inup to 85% of all in-hospital resuscitation attempts,214 but accurate prehospital diagnosis of acute pulmonary embolism is particularly challenging.212, 216 The 2014 European Society of Cardiology Guidelines on the diagnosis and management of acute pulmonary embolism define ‘confirmed pulmonary embolism’ as a probability of pulmonary embolism high enough to indicate the need for specific treatment.208
Clinical history and assessment, capnography and echocardiography (if available) can all assist in the diagnosis of acute pulmonary embolism duringCPR with varying degrees of specificity and sensitivity:
- Common symptoms preceding cardiac arrest are sudden onset of dyspnoea, pleuritic or substernal chest pain, cough, haemoptysis, syncope and signs of DVTin particular (unilateral low extremity swelling).208 However, pulmonary embolism may not be symptomatic until it presents as sudden cardiac arrest.217
- Obtain information about past medical history, predisposing factors, and medication that may support diagnosis of pulmonary embolism, although none of these are specific, e.g.208
- Previous pulmonary embolism or DVT
- Surgery or immobilisation with in the past four weeks
- Active cancer
- Clinical signs of DVT
- Oral contraceptive use or hormone replacement therapy.
- Long-distance flights
In as many as 30% of the patients with pulmonary embolism, no risk factors are apparent.218
- If a 12-lead ECG can be obtained before onset of cardiac arrest, changes indicative of right ventricular strain may be found:
- Inversion of Twaves in leads V1–V4
- QR pattern in V1
- S1Q3T3 pattern(i.e. aprominent Swave in lead I, aQ wave and inverted Twave in leadIII)
- Incomplete or complete right bundle-branch block208, 219
- Cardiac arrest commonly presents as PEA.211
- Low ETCO2 readings(about 1.7 kPa/13 mm Hg) while performing high quality chest compressions may support a diagnosis of pulmonary embolism, although it is a non-specific sign.209
- Consider emergency echo cardiography performed by a qualified sonographer as an additional diagnostic tool to identify pulmonary embolism if it can be performed without interruptions to chest compressions, e.g. during rhythm check. Echocardiographic findings are evident after acute obstruction of more than 30% of the pulmonary arterial tree.220 Common echocardiographic findings are an enlarged right ventricle with a flattened interventricular septum,221, 222 but absence of these features does not exclude pulmonary embolism.223 Signs of right ventricular overload or dysfunction may also be caused by other cardiac or pulmonary disease.224
- More specific diagnostic methods, e.g. D-dimertesting,(computed tomographic) pulmonary an giography, lungscintigraphy, or magnetic resonance an giography, are not recommended for a cardiac arrest situation.
Modifications to cardiopulmonary resuscitation. A meta-analysis, which included patients with pulmonary embolism as a cause of cardiac arrest, concluded that fibrinolytics increased the rate of ROSC, survival to discharge and long term neurological function.225 A subgroup analysis of patients treated with thrombolytics compared with placebo in a randomised controlled trial215 did not prove survival difference. However, this study was not designed for treatment of pulmonary embolism and not powered to reach significance in this small subgroup. Some other non-randomised studies have also documented use of thrombolytics in the treatment of cardiac arrest due to acute pulmonary embolism, but evidence for improved neurologically intact survival to hospital discharge is limited.211, 226
In a cardiac arrest presumed to be caused by acute pulmonary embolism, follow the standard guidelines for ALS (see adult advanced lifesupport).168 The decision to treat for acute pulmonary embolism must be taken early, when a good outcome is still possible.The following treatment modifications are recommended:
- Consider administration of fibrinolytic therapy when acute pulmonary embolism is a known or suspected cause of cardiac arrest. Ongoing CPR is not a contra indication to fibrinolysis. Despite increased risk of severe bleeding, fibrinolys is may be an effective treatment, which can be initiated without delay, even outside specialised healthcare facilities. The potential benefit of fibrinolysis in terms of improved survival outweighs potential risks in a location where no alternative exists, e.g. in the prehospital setting.211, 227–231
- Once a fibrinolytic drug is administered, continue CPR for at least 60–90min before terminating resuscitation attempts.227, 232 Survival and good neurological outcome have been reported in cases requiring in excess of 100 min of CPR.233
- Consider the use of a mechanical chest compression device when maintenance of high quality chest compressions is needed for a prolonged time.
Extra corporeal CPR. Some observational studies suggest the use of extracorporeal life support (ECLS) if cardiac arrest is associated with pulmonary embolism.234, 235 The implementation of ECLS requires considerable resource and training. Its use should be considered as a rescue therapy for those patients in whom initial ALS measures are unsuccessful and/or to facilitate pulmonary thrombectomy.
Surgical embolectomy and mechanical thrombectomy. Survival of patients who underwent surgical embolectomy during CPR due to pulmonary embolism was reported as 13% and 71% in two case series,229, 236 but these results were not compared with standard treatment. Routine use of surgical embolectomy or mechanical thrombectomy for cardiac arrest from suspected pulmonary embolism is not recommended, but these methods may be considered when pulmonary embolism is the known cause of cardiac arrest.
Percutaneous pulmonary thrombectomy. In one case series, percutaneous pulmonary thrombectomy during CPR was successful in six of seven patients,237, 238 but larger studies are needed to validate this method.
Post-resuscitation care. In patientswith sustained ROSC, exclude intra-abdominal and intra-thoracic CPR-related injuries, especially if a mechanical chest compression device was used simultaneously with administration of fibrinolytics.239–241 Attempt to identify and treat the original cause of the pulmonary embolism. Evaluate the risks of a further pulmonary embolism and treat accordingly.
Coronary thrombosis
Coronary heart disease is the most frequent cause of OHCA. The periresuscitation management of acute coronary syndromes is addressed in a separate chapter (see Section 8 Initial management of acute coronary syndromes).242 In cardiac arrest centres, coronary artery occlusion or high degree stenoses can be identified and treated. Of all patients in OHCA, however, at least half are not transported to hospital when ROSC is not achieved (see Section 10 Ethics of resuscitation and end-of-life decisions).243 Although proper diagnosis of the cause may be difficult in a patiental ready in cardiac arrest, if the initial rhythm is VF it is most likely that the cause is coronary artery disease with an occluded large coronary vessel.
Consider transportation to hospital with ongoing CPR if treatment options are available that cannot be applied in the prehospital setting, such as immediate coronary angiography, primary percutaneous coronary intervention (PPCI) or other interventions such as (more rarely) pulmonary embolectomy (see pulmonary embolism). The decision to transport is complex and may depend on local circumstances.Prehospital initiation of extra corporeal cardio pulmonary lifesupport (ECLS) requires specialised expertise and its feasibility on a wide-scale has not been established.244–246
Mechanical chest compression devices maintain high quality CPR during transport and PCI (see cardiac arrest in HEM Sandair ambulances).247, 248 There is limited evidence for recommending routine transport to hospital with ongoing CPR.The decision will depend on patient selection, availability of optimal methods for mechanical or circulatory support during and after transport to the hospital, management of underlying pathology, treatment after ROSC, complication rate and outcome. There are no large outcome studies available, but small case series suggest benefit in selected cases.249 Before definitive recommendations can be made, controlled studies are needed.250
Transport with ongoing CPR and immediate access to the catheterisation laboratory may be considered if a prehospital and in-hospital infrastructure is available with teams experienced in mechanical or haemodynamic support and rescue PPCI with ongoing CPR. Excellent cooperation is required between prehospital and in-hospital teams. A decision to transport with ongoing CPR should take into consideration a realistic chance of survival(e.g. witnessed cardiac arrest with initial shockable rhythm (VF/pVT) and bystander CPR). Intermittent ROSC also strongly favours a decision to transport.251
Toxins
General considerations Introduction. Overall, poisoning rarely causes cardiac arrest or death,252 but hospital admissions are common, accounting for as many as 140,000 admissions each year in the UK.252 Poisoning by therapeutic or recreational drugs and by household products are the main reasons for hospital admission and poison centre calls. Inappropriate drug dosing, drug interactions and other medication error scanalso cause harm. Accidental poisoning is commonest in children. Homicidal poisoning is uncommon. Industrial accidents, warfare orterrorism canalso cause exposure to toxins.Evidence for treatment consists primarily of animal studies, case reports and small case series.253–255
Prevention of cardiac arrest. Assess the patient using systematic ABCDE approach. Airway obstruction and respiratory arrest secondary to a decreased conscious level is a common cause of death after self-poisoning (benzodiazepines, alcohol, opiates, tricyclics, barbiturates).256, 257 Early tracheal intubation of unconscious patients by trained personnel may decrease the risk of aspiration. Drug-induced hypotension usually responds to IV fluids, but occasionally vasopress or support (e.g. noradrenaline infusion) is required. Measure electrolytes(particularly potassium), blood glucose and arterial blood gases. Retain samples of blood and urine for analysis. Patients with severe poisoning should be cared for in a critical care setting.257 Modifications to resuscitation.
- Have a low threshold to ensure your personal safety where there is a suspicious cause or unexpected cardiac arrest. This is especially so when there is more than one casualty.
- Avoid mouth-to-mouth breathing in the presence of chemicals such as cyanide, hydrogen sulphide, corrosives and organophosphates.
- Treat life-threatening tachyarrhythmias with cardio version according to the periarrestarrhythmia guidelines(see adult advanced life support).168 This includes correction of electrolyte and acid-base abnormalities (see hypo-/hyperkalaemia and other electrolyte disorders).
- Try to identify the poison(s). Relatives, friends and ambulance crews can provide useful information. Examination of the patient may reveal diagnostic clues such as odours, needle marks, pupil abnormalities, and signs of corrosion in the mouth.
- Measure the patient’s temperature because hypo- or hyperthermia may occur after drug overdose(seehypo-/hyperthermia).
- Be prepared to continue resuscitation for a prolonged period, particularly in young patients, as the poison may be metabolised or excreted during extended resuscitation measures.
- Alternative approaches which may be effective in severely poisoned patients include:higher doses of medication than in standard protocols (e.g. high-dose insulin euglycemia)258; non-standard drug therapies (e.g. IV lipid emulsion)259–262; prolonged CPR, extracorporeal life support (ECLS),263, 264 and haemodialysis.
- Consult regional or national poisons centres for information on treatment of the poisoned patient.The International Programme on Chemical Safety (IPCS) lists poison centres on its website: http://www.who.int/ipcs/poisons/centre/en/.
- On-line databases for information ontoxicology and hazardous chemicals may be helpful: http://toxnet.nlm.nih.gov/. Specific therapeutic measures There are few specific therapeutic measures for poisoning that are useful immediately and improve outcomes: decontamination, enhancing elimination, and the use of specific antidotes.265–267 Many of these interventions should be used only based on expert advice.For up-to date guidance in severe or uncommon poisonings, seek advice from a poisons centre.
Decontamination. Decontamination is a process of removal of the toxin from the body determined by the route of exposure:
- For dermal exposures initial management consists of clothing removal and copious irrigation with water, except in case of reactive alkali metals that can ignite.
- Routine use of gastric lavage for gastrointestinal decontamination is no longer recommended. In the rare instances (e.g. lethal ingestion with recent exposure), it should only be performed by individuals with proper training and expertise. Gastric lavage may be associated with life-threatening complications, e.g. aspiration pneumonitis, aspiration pneumonia, esophageal or gastric perforation, fluid and electrolyte imbalances, arrhythmia. It is contraindicated if the airway is not protected and if a hydrocarbon with high aspiration potential or a corrosive substance has been ingested.267, 268
- The preferred method of gastro intestinal decontamination in patients with an intact or protected airway is activated charcoal. It is most effective if given within 1h of the time of the ingestion.269 Activated charcoal does not bind lithium, heavymetals and toxic alcohols. Most common side effects are vomiting and constipation. The evidence that active charcoal improves outcome is limited.257
- Based mainly on volunteer studies, consider whole-bowel irrigation in potentially toxic ingestions of sustained-release or enteric-coated drugs particularly for those patients presenting later than 2h after drug ingestion when activated charcoal is less effective. It may be also used for the removal of substantial amounts of iron, lithium, potassium, or packets of illicit drugs. Whole-bowel irrigation is contra indicated in patients with bowel obstruction, perforation, ileus, and haemodynamic instability.270
- Avoid routine administration of laxatives (cathartics) and do not use emetics (e.g. ipecac syrup).271–273 Enhanced elimination. Modalities removing a toxin from the body once it has been absorbed include multiple-dose activated charcoal (MDAC), urinary alkalinisation and extra corporeal elimination techniques:
- MDAC, multiple doses of activated charcoal administered over several hours, can increase certain drug elimination.274, 275 Give an initial dose of 50–100g in adults(25–50g in children).
- Urinary alkalinisation (urine pH≥7.5) involves an IV sodium bicarbonate infusion. It is most commonly performed in patients with salicylate in toxication who do not need dialysis. Consider urine alkalinisation with high urine flow (about 600 mLh− 1 ) in severe poisoning by phenobarbital and herbicides, e.g. 2,4- dichl or ophenoxy acetic acid or methylchlor ophenoxy propionic acid (mecoprop). Hypokalaemia is the most common complication.265
- Haemodialys is removes drugs or metabolites with low molecular weight, low protein binding, small volumes of distribution and high water solubility. In case of hypotension, use continuous veno-venous hemofiltration(CVVH) or continuous veno-venous haemodialysis (CVVHD) alternatively.257
Specific poisons
These guidelines address only some of the more common poisons causing cardiac arrest.
Benzodiazepines. Overdose of benzodiazepines can cause loss of consciousness, respiratory depression and hypotension. Flumazenil, a competitive antagonist of benzodiazepines, may be used for reversal of benzodiazepines edation when there is no history or risk of seizures. Reversal of benzodiazepine intoxication with flumazenil can be associated with significant toxicity (seizure, arrhythmia, hypotension, and withdrawal syndrome) in patients with benzodiazepine dependence or co-ingestion of proconvulsant medications such as tricyclic antidepressants.256–278
The routine use of flumazenil in the comatose overdose patient is not recommended. There are no specific modifications to the ALS algorithm required for cardiac arrest caused by benzodiazepines.278–282
Opioids. Opioid poisoning causes respiratory depression followed by respiratory insufficiency or respiratory arrest. The respiratory effects of opioids are reversed rapidly by the opiate antagonist naloxone. In severe respiratory depression caused by opioids, thereare fewer adverse events when airway opening, oxygen administration and ventilation are carried out before giving naloxone283–289; The use of naloxone can prevent the need for intubation. The preferred route for giving naloxone depends on the skills of the rescuer:intravenous(IV), intramuscular(IM), subcutaneous (SC), intraosseous (IO) and intranasal (IN) routes are all suitable.290, 291
The non IV routes can be quicker because time is saved in not having to establish IV access, which may be difficult in an IV drug abuser. The initial doses of naloxone are 0.4–2mg IV, IO, IMorSC, and may be repeated every 2–3 min. Additional doses may be needed every 20–60 min. Intranasal dosing is 2 mg IN (1 mg in each nostril) which may be repeated every 5 min. Titrate the dose until the victim is breathing adequately and has protective airway reflexes. Large opioid overdoses may require a total dose of up to 10 mg of naloxone.283–285, 290–300 All patients treated with naloxone must be monitored.
Acute withdrawal from opioids produces a state of sympathetic excess and may cause complications such as pulmonary oedema, ventricular arrhythmias and severe agitation. Use naloxone reversal of opioid intoxication with caution in patients suspected of opioid dependence. There are no data on the use of any additional therapies beyond standard ALS guidelines in opioid-induced cardiac arrest. In respiratory arrest there is good evidence for the use of naloxone, but not for any other adjuncts or changes in interventions.284
Tricyclic antidepressants. This section addresses both tricyclic and related cyclic drugs (e.g. amitriptyline, desipramine, imipramine, nortriptyline, doxepin, and clomipramine). Self-poisoning with tricyclic antidepressants is common and can cause hypotension, seizures, coma and life-threatening arrhythmias. Cardiactoxicity mediated by anticholinergic and Na+ channel-blocking effects can produce a wide complex tachycardia (VT). Hypotension is exacerbated by alpha-1 receptor blockade. Anticholinergic effects include mydriasis, fever, dry skin, delirium, tachycardia, ileus, and urinary retention. Most life-threatening problems occur within the first 6h after ingestion.301–303 Awidening QRS complex (>100ms) and right axis deviation indicates a greater risk of arrhythmias.304–306 Give sodium bicarbonate (1–2 mmol kg− 1 ) for the treatment of tricyclic-induced ventricular arrhytmias.307–312 While no study has investigated the optimal target arterial pH with bicarbonate therapy, a pH of 7.45–7.55 is recommended.255, 257 Administration of bicarbonate may resolve arrhythmias and reverse hypotension even in the absence of acidosis.312
Intravenous lipid infusions in experimental models of tricyclic toxicity have suggested benefit but there are few human data.313, 314 Anti-tricyclic antibodies have also been beneficial in experimental models of tricyclic cardiotoxicity.315–320 One small human study provided evidence of safety but clinical benefit has not been shown.321 There are no randomised controlled trials evaluating conventional versus alternative treatments for cardiac arrest caused by tricyclic toxicity.One small case series show edim provement with the use of sodium bicarbonate but the concomitant use of physostigmin prevents the ability to generalise its results.322
Cocaine. Sympathetic overstimulation associated with cocaine toxicity can cause agitation, tachycardia, hypertensive crisis, hyperthermia and coronary vasoconstriction causing myocardial is chaemia with angina. In patients with severe cardiov asculartoxicity, alphablockers (phentolamine),323 benzodiazepines (lorazepam, diazepam),324, 225 calcium channel blockers (verapamil),326 morphine,327 and sublingual nitroglycerine328, 339 may be used as needed to control hypertension, tachycardia, myocardialischaemia and agitation. The evidence for or against the use of beta-blocker drugs,330–333 including those beta-blockers with alpha blocking properties (carvedilol and labetolol) is limited.334–336 The optimal choice of anti-arrhythmic drug for the treatment of cocaine-induced tachyarrhythmias is not known. If cardiac arrest occurs, follow standard resuscitation guidelines.337
Local anaesthetics. Systemic toxicity of local anaesthetics involves the central nervous and cardiovascular systems. Severe agitation, loss of consciousness, seizures, bradycardia, asystole or ventricular tachyarrhythmias can all occur.
Toxicity typically occurs in the setting of regional anaesthesia, when a bolus of local anaesthetic in advertently enters an arteryor vein (see perioperative cardiac arrest).
Although there are many case reports and case series of patients who were resuscitated after administration of IV lipid emulsion, evidence for its benefit in treating local anaesthetic-induced cardiac arrest is limited. Despite the paucity of data, patients with both cardiovascular collapse and cardiac arrest attributable to local anaesthetic toxicity may benefit from treatment with intravenous 20% lipidemulsion in addition to standard ALS.338–352 Give an initial intravenous bolus injection of 20% lipid emulsion 1.5 mL kg−1 over 1 min followed by an infusion at 15 mL kg−1 h−1. Give up to a maximum of two repeat boluses at 5-min intervals and continue until the patient is stable or has received up to a maximum cumulative dose of 12mL kg−1 of lipid emulsion.259– 262,353 Standard cardiac arrests drugs(e.g.adrenaline) should be given according to ALS guidelines, although animal studies provide inconsistent evidence for their role in local anaesthetic toxicity.349, 352, 354–356
Beta-blockers. Beta-blocker toxicity causes brady arrhythmias and negative inotropic. effects that are difficult to treat, and can lead to cardiac arrest.
Evidence for treatment is based on case reports and animal studies. Improvement has been reported with glucagon (50–150 mcg kg−1),357–370 high-dose insulin and glucose,371–373 lipid emulsions,374–377 phosphodiesterase inhibitors,378,379 extracorporeal and intra-aortic balloon pump support,380–382 and calcium salts.258,383
Calcium channel blockers. Calcium channel blocker overdose is emerging as a common cause of prescription drug poisoning deaths.384,385 Overdose of short-acting drugs can rapidly progress to cardiac arrest. Overdose by sustained-release formulations can result in delayed onset of arrhythmias, shock, and sudden cardiac collapse. The treatment for calcium channel blocker poisoning is supported by low- quality evidence.386
Give calcium chloride 10% in boluses of 20 mL (or equivalent dose of calcium gluconate every 2–5 min in severe bradycardia or hypotension followed by an infusion if needed.255, 257, 258, 386, 387
While calcium in high doses can overcome some of the adverse effects, it rarely restores normal cardiovascular status. Haemodynamic instability may respond to high doses of insulin (1 unit kg−1 followed by an infusion of 0.5–2.0 units kg−1 h−1) give with glucose supplementation and electrolyte monitoring in addition to standard treatments including fluids and vasopressors (e.g. dopamine, norepinephrine, vasopressin).386–398 Extracorporeal life support(ECLS) was associated with improved survival in patients with severe shock or cardiac arrest at the cost of limbischaemia, thrombosis, and bleeding.264 Studies on decontamination, 4-aminopyridine, atropine, glucagon, pacemakers, levosimendan, and plasma exchange reported variable results.386
Digoxin. Although cases of digoxin poisoning are fewer than those involving calcium channel and beta-blockers, the mortality rate from digoxin is far greater. Other drugs including calcium channel blockers and amiodarone can also cause plasma concentrations of digoxin to rise. Atrioventricular conduction abnormalities and ventricular hyper excitability due to digoxin toxicity can lead to severe arrhythmias and cardiac arrest.
Specific antidote therapy with digoxin-specific antibody fragments (digoxin- Fab) should be used if there are arrhythmias associated with haemodynamic instability.257,399–401 Digoxin-Fab therapy may also be effective in poisoning from plants (e.g.oleander) and Chinese herbal medications containing cardiac glycosides.399,402,403 Digoxin-Fab interfere with digoxin immunoassay measurements and can lead to overestimation of plasma digoxin concentrations. In acute poisoning, give an initial bolus of 2 vials digoxin-Fab (38mg pervial) and repeat dose if necessary.401 In a cardiac arrest, consider administration of 2 up to 10 vials IV over 30 min.
Cyanides. Cyanide is generally considered to be a rare cause of acute poisoning; however, cyanide exposure occurs relatively frequently in patients with smoke inhalation from residential or industrial fires. Cyanides are also used in several chemical and industrial processes. Its main toxicity results from inactivation of cytochrome oxidase (at cytochrome a3), thus uncoupling mitochondrial oxidative phosphorylation and inhibiting cellular respiration, even in the presence of adequate oxygen supply. Tissues with the highest oxygen needs (brain and heart) are the most severely affected by acute cyanide poisoning.
Patients with severe cardiovascular toxicity (cardiac arrest, cardiovascular instability, metabolicaci dosis, or altered mental status) caused by known or suspected cyanide poisoning should receive cyanide antidote therapy in addition to standard resuscitation, incl. oxygen. Initial therapy should include acyanidescavenger (either hydroxocobalamin 100 mg kg−1 IV or a nitrite – i.e. IV sodium
nitrite and/or inhaled amyl nitrite), followed as soon as possible by IV sodium thiosulfate.404–410 Hydroxocobalamin and nitrites are equally effective but hydroxocobalamin is safer because it does not cause methaemoglobin formation or hypotension.411–413
In the case of cardiac arrest caused by cyanide, standard treatment fails to restore spontaneous circulation as long as cellularrespiration is blocked. Antidote treatment is needed for reactivation of cytochromeoxidase.
Carbonmonoxide. Carbon monoxide poisoning is common. There were about 25,000 carbon monoxide related hospital admissions reported yearly in the United States.414 Carbon monoxide levels do not correlate with the presence or absence of initial symptoms or with later outcomes.415 Patients who develop cardiac arrest cause by carbon monoxide rarely survive to hospital discharge, even if ROSC is achieved.413,416
Give oxygen as soon as possible. The use of hyperbaric oxygen has been used to treat carbon monoxide exposure in order to reduce the incidence of adverse neurologic outcomes.417 However, two Cochrane reviews failed to demonstrate convincing benefit from hyperbaric oxygen therapy for patients with carbon monoxide poisoning.416,418 The role of carbon monoxide in nitric oxide release, reactive oxygen species formation, and its direct action onion channels may be more significant than its higher affinity for haemoglobin, which is treated by oxygen therapies.419 There is unproven benefit for transporting critically ill post-arrest patients to a hyperbaric facility and such decision must be considered on a case-by-case basis.413,416,418,419 Patients who develop myocardial injury caused by carbon monoxide have an increased risk of cardiac and all-cause mortality lasting at least seven years after the event; it is reasonable to recommend cardiology follow-up for these patients.413,420,421
B–SPECIALENVIRONMENTS
Cardiac arrest in healthcare facilities
Perioperative cardiac arrest
Introduction. Although the safety of routine surgical procedures has increased over recent decades, the greater number of procedures being performed, particularly in more elderly patients and in emergency situations has resulted in a broadly stable incidence of perioperative cardiac arrests over the past decade.
Although the features of perioperative cardiac arrest are often different to those of cardiac arrests occurring in the general hospital population, the principles of treatment are similar. Perioperative cardiac arrest may be caused by the underlying condition being treated, physiological effects of the surgery, anaesthetic drugs and fluids, complications relating to existing co-morbidities, or adverse events.
Epidemiology. The overall incidence of perioperative cardiac arrest ranges from 4.3 to 34.6 per 10,000 procedures.422–424 This wide range reflects differences in case-mix (some include neonates and/or cardiac surgery) and in the definition of perioperative. The incidence is higher in high-risk groups such asthe elderly where it has been reported as 54.4 per 10,000 cases425 and in patients undergoing emergency surgery where an incidence of 163 per 10,000 cases has been reported.426 Young age (<2 years old), cardiovascular and respiratory comorbidities, increasing American Society of Anesthesiologists (ASA) physical status classification, preoperative shock, and surgery site have all been identified as risk factors for perioperative cardiac arrest.426
The incidence of cardiac arrest attributable primarily to anaesthesia is a relatively small proportion of this overall incidence and in recent studies is estimated to be 1.1– 3.26per10,000 procedures.425,427,428 Overall survival from perioperative cardiac arrest is higher than from OHCA, with survival to hospital discharge rates of 30–36.6% being reported recently.422,424,428
General versus regional anaesthesia. The incidence of perioperative cardiac arrest during general anaesthesia(GA) is higher than that of regional anaesthesia(RA). The incidence of cardiac arrest for patients receiving general anaesthesia in a study from theMayo Clinic was higher(almost 3 times higher, at 4.3 per 10,000)than that for those receiving regional anaesthesia or monitored anaesthesia care. The incidence however decreased significantly over a 10-year period.423
Causes of cardiac arrest. Overall causes of cardiac arrest havebeen identified as:
- Hypovolaemia (e.g.bleeding).
- Cardiac-related.
- Other:
- Drug-induced (e.g. muscle relaxants).
- Anaphylaxis(drugs, blood products).
- Airway loss
- Ventilation failure
- Anaphylaxis (drugs, blood products).
The commonest cause of anaesthesia-related cardiac arrest involves airway management.427,428 Failure of ventilation, medication-related events, complications associated with central venousaccess, and perioperative myocardial infarction are also common.423,429 In children, airway obstruction from laryn-gospasm, hypovolaemia from blood loss and hyperkalemia from transfusion of stored blood are additional causes.430
Cardiac arrest caused by bleeding had the highest mortality in non-cardiac surgery, with only 10.3% of these patients surviving to hospital discharge.423 The primary arrest rhythms during perioperative cardiac arrest recorded in the Mayo Clinic series were asystole in 41.7%, VF in 35.4%, PEA in 14.4% and unknown in 8.5%. Contrary to studies of cardiac arrest in general, therhythm associated with the best chance of survival to hospital discharge was asystole (43% survival).423,431
Management of perioperative cardiac arrest. Patients in the operating room are normally fully monitored and, as such, there should be little or no delaying diagnosing cardiac arrest. High-risk patients will often have invasive blood pressure monitoring, which is invaluable in the event of cardiac arrest. If cardiac arrest is a strong possibility, apply self-adhesive defibrillation electrodes before induction of anaesthesia, ensure adequate venous access and prepare resuscitation drugs and fluids. Use fluid warmers and forced air warmers to limit perioperative hypothermia and monitor the patient’s temperature.
In the event of cardiac arrest, follow the ALS algorithm, but with appropriate modifications. Adjust the position and height of the operating table or trolley to optimise delivery of chest compressions. CPR is optimal in the supine position, but is possible in patients who are prone and where immediate turning to a supine position is not possible.432,433 Risk factors for cardiac arrest in prone patients include cardiac abnormalities in patients undergoing major spinal surgery, hypovolaemia, air embolism, wound irrigation with hydrogen peroxide, occludedvenous return.
Identification of causes. In many cases of perioperative cardiac arrest, physiological deterioration is gradual and the cause of the cardiac arrest is known and hence the arrest anticipated. In those where this is not the case, follow the standard ABC algorithm to identify and treat reversible causes. If patients deteriorate, call for senior help immediately. Inform the perioperative team of the deterioration and possible impending cardiac arrest, ensuring that sufficient skilled assistance is present.
C Catastrophic haemorrhage is usually obvious, but may be occult it if involves bleeding into body compartments (abdomen, chest) or into soft tissues in patients with multiple limb fractures. Pelvic and retroperitoneal haemorrhage can also cause rapid hypovolaemia and should be excluded, e.g. by ultra sound if pre-operative haemodynamic instability.In cases where direct surgical intervention is unable to control haemorrhage, early interventional radiography should be considered.
- Loss of the airway is a common cause of perioperative
Assess the airway carefully before induction of anaesthesia. Prepare all equipment, including suction and an operating table or trolley that can be tipped head-down (Trendelenburg position). Ensure that difficult airway equipment is immediately available, and brief the team on a failed intubation drill if appropriate. Always use waveform capnography.
Children are particularly prone to loss of the airway from laryngospasm; ensure an appropriate neuromuscular blocker is available and give before significant hypoxaemia has occurred in order to break the laryngospasm.
- Undiagnosed tension pneumothorax is a readily treatable cause of Although usually associated with trauma, considerearly in the management of all patients who arrest, particularly those with chronic obstructive pulmonary diseaseand severe asthma. A sudden increase in airway pressures may indicatea tension pneumothoraxor problems with the breathing tubing, but also consider asthma and anaphylaxis.
- Cardiovascular collapse has several causes, but int he context of perioperative cardiac arrest, common causes include hypovolaemia, anaphylaxis, andvagal stimulation. Use of transthoracic echocardiography is a useful tool to exclude cardiac tamponade (if suspected) and to assess myocardial contractility and
Anaphylaxis. The incidence of immune-mediated anaphylaxis during anaesthesia ranges from 1 in 10,000 to 1 in 20,000.434
Neuromuscular blocking drugs are the commonest cause, being associated. with 60% of cases. The associated morbidity and mortality are high, particularly if there are delays in the diagnosis and management. Initial management of anaphylaxis follows the ABC approach and the management principles outlined in the chapter on anaphylaxis. Adrenaline is the most effectived rug in anaphylaxis an disgiven as early as possible. It is appropriate for anaesthetists to give adrenaline by the intravenous route. Repeated doses may be necessary.
If cardiac arrest ensues despite correct treatment for the anaphylaxis (see anaphylaxis), continue resuscitation using the standard ALS algorithm (see adult advanced life support).168
Systemic toxicity of local anaesthetics. Cardiac arrest is a rare but well recognised complication of local anaesthetic (LA) overdose, especially following inadvertent intravascular injection. Direct action of the LA on cardiac myocytes causes cardiovascular collapse, usually within 1–5 min of injection, but onset may range from 30s to as long as 60min.435 Significant hypotension, dysrhythmias, and seizures are typical manifestations, but diagnosis may be one of exclusion.436 IV lipid therapy has been used as a rescue therapy to treat cardiovascular collapse and cardiac arrest, but its efficacy is debated.437
In the absence of documented harm, guidelines recommend that 20% lipidemulsion should be available for use wherever. patients receive large doses of LA(e.g. operating rooms, labourwards and the emergency department).353,438 Stop injecting the LA and call for help. Secure and maintain the airway and, if necessary, intubate. Give 100% oxygen and ensure adequate ventilation (hyperventilation may help by increasing plasma pH in the presence of metabolic acidosis). Control seizures using a benzodiazepine, thiopental or propofol. Give an initial IV bolus injection of 20% lipid emulsion at 1.5 mL kg−1 over 1min and then start an infusion at 15 mL kg−1 h−1. If ROSC has not been achieved at 5 min, double the rate of lipid infusion and give a maximum of two additional lipid boluse sat 5-min intervals until ROSC has been achieved. Do not exceed a maximum cumulative dose of 12 mL kg−1.259,260
Diagnosis of cardiac arrest. Asystole and ventricular fibrillation (VF) will be detected immediately, but the onset of PEA might not be so obvious– loss of the pulse oximeter signal and very low end-tidal CO2-values will be good clues and should provoke a pulsecheck. Do not waste time attempting to obtain a non-invasive blood pressure measurement.
Management of cardiac arrest. The management of a cardiac arrest follows the principles of the ALS algorithm. Chest compression in the prone position can be achieved with or without sternal counter-pressure. In one study of prone CPR with sternal counter pressure(provided by a sandbag) versusstandard CPR, higher mean arterial pressures were achieved with the prone technique.439 Consider open cardiac compressions in patients where the thoraxis open or the heart can be easily accessed.
Ventricular fibrillation. In the case of VF, call for a defibrillator. If one is not immediately available, apply a precordial thump. If that is unsuccessful, give chest compressions and ventilation until the defibrillator arrives. Look for reversible causes immediately– hypoxaemia and hypovolaemia will be the most common in this setting.
Asystole/extreme bradycardia. Stop any surgical activity likely to be causing excessive vagal activity– if this is the likely cause– give 0.5 mg atropine IV/IO(not 3 mg). Start CPR and immediately look for other reversible causes. Exclude a completely straight line, which suggests an ECG monitoring lead has become detached.
Pulseless electrical activity. Start CPR while looking quickly for reversible causes of PEA. Give fluid unless you are certain that the intravascular volume is adequate.Stopadministrationoftheanaesthetic.Whileavasopressorwillberequired, in these circumstances 1mg of adrenaline (as directed by the standard ALS guidelines) may be excessive. Give a smaller dose (e.g. 1mcg kg−1) of adrenaline, or another vasopress or initially; if this fails to restore the cardiac output, increase the dose while continuing to perform chest compressions and ventilation.
Monitoring and feedback during CPR.Unlike OHCAs where monitoring if often limited, patients arresting in the perioperative period can often be monitored with a greater degree of precision.
Monitoring enables assessment of rescuer performance and patient response:
- Rescue CPR performance
Feedback sensors (e.g. accelerometers) improve the delivery of effective chest compressions and enable the rescuer to tailor their performance accordingly. Their use should be considered whenever available. Performance feedback can be obtained from invasive and non-invasive patient monitoring and the rescuer should have direct visualisation of monitors displaying these data.
- Patient response
Monitoring of the patient requires adequate lighting and patient exposure. Non- invasive blood pressure is unlikely to be of assistance until ROSC is achieved, but in patients with invasive arterial monitoring, aim for a diastolic blood pressure >25 mm Hg,440 titrating it to this level (after chest compressions are optimised) by administration of a vasopressor, if necessary. This goal is based on expert consensus derived from experimental and limited clinical data.441–443
Waveform capnography is a minimum monitoring standard during anaesthesia and therefore immediately available during a perioperative cardiac arrest. In addition to its use for patients with tracheal intubation where it is particularly valuable to confirm correct tracheal tube placement, it may also be used in patients with supraglottic airway devices (although an airleak may limit quantitative evaluation). An end-tidal carbon dioxide (ETCO2) value <1.4 kPa/10 mm Hg suggests a low cardiac output and rescuers may be able to adjust their technique to optimise this variable. An abrupt sustained increase to a normal value(4.7–5.4kPa/35–40mmHg) or even higher may be an indicator of ROSC. Optimise CPR to achieve anETCO2 >2.7 kPa/20 mmHg, while ventilating the lungs at about 10 breaths min−1, with only minimal chest rise).440
Team working. Every resuscitation event should have a designated team leader who directs and coordinates all staff and the components of the resuscitation, with a central focus on delivering high-quality CPR. Stop operative surgery unless it is addressing a reversible cause of the cardiac arrest. Patient access and resuscitation tasks may necessitate covering the surgical field and withdrawing the surgical team from the patient. Prioritise team tasks, ensure good quality basic lifesupport
(BLS), identify reversible causes and avoid non-priority tasks.440 If the patient is not responding to resuscitative efforts (i.e. ETCO2 <2.7 kPa/20 mmHg), try to improve the quality of CPR by optimising:(1) compression fraction,(2) compression rate,(3) compression depth,(4) leaning, and(5) by avoiding of excessive ventilation.440
Post-resuscitation care. Depending on the circumstances, patients successfully resuscitated after a very brief period of cardiac arrest, e.g. asystole from excessive vagal stimulation may not require anything more than standard post-operative care. All those resuscitated successfully after longer periods of cardiac arrest will require admission to an ICU–unless further active treatment is deemed inappropriate. In most circumstances, anything but immediately life-saving surgery should be abandoned to enable admission to ICU for post-resuscitation care. Patients resuscitated after a prolonged period of cardiac arrest may develop a marked systemic inflammatory response syndrome (SIRS) with the risk of multiple organ failure. They will require optimisation of mean arterial pressure, oxygenation and ventilation. These patients may have sustained a significant cerebral insult. Some may be suitable for targeted temperature management, but this requires careful consideration, given the lack of data on this therapy in the setting of perioperative cardiac arrest. Active bleeding would certainly be a contraindication to induced mild hypothermia but, at the very least, prevent fever in all cases. Avoidance of hyperthermia, from overwarming or a post-cardiac arrest syndrome444 is important to optimise neurological recovery.
Do not attempt resuscitation decisions. Patients with DNAR decisions presenting for surgery present a dilemma for the anaesthetist. The anaesthetic will induce cardiovascular instability, many of the routine interventions undertaken could be considered as resuscitative, and the chances of surviving a perioperative cardiac arrest are better than those from in-hospital cardiac arrest in general. Consider each case on its individual merits and discuss with the patient and/or relatives. Some patients may wish a DNAR decision to remain valid despite the increased risk of a cardiac arrest and the presence of potentially reversible causes; others will request that the DNAR decision is suspended temporarily. Discuss and agree on the time at which the DNAR decision is reinstated.445
Cardiac arrest following cardiac surgery
Introduction. Cardiac arrest following major cardiac surgery is relatively common in the immediate post-operative phase, with a reported incidence of 0.7–8%.446–455 It is usually preceded by physiological deterioration,456 although it can occur suddenly in stable patients.452 There are usually specific causes of cardiac arrest, such as tamponade, hypovolaemia, myocardialischaemia, tension pneumothorax, or pacing failure. These are all potentially reversible and if treated promptly cardiac arrest after cardiac surgery has a relatively high survival rate. Key to the successful resuscitation of cardiac arrest in these patients is recognition of the need to perform emergency resternotomy early, especially in the context of tamponade or haemorrhage, where external chest compressions may be ineffective.
Starting CPR. If VF or asystole is diagnosed, immediately administer external defibrillation or emergency temporary pacing at maximum amplitude. Otherwise start external chest compressions immediately in patients who arrest with monitoring indicating no output. Verify the effectiveness of compressions by looking at the arterial trace, aiming to achieve a systolic blood pressure >60 mmHg [Society of Thoracic Surgeons (STS) Clinical Practice guidelines in preparation – personal communication from Joel Dunning] and a diastolic blood pressure >25 mm Hg440 at a rate of 100–120min−1. Inability to obtain this goal with external chest compressions indicates that cardiac tamponade or extreme hypovolaemia is likely and emergency resternotomy should be performed.
Consider other reversible causes:
- Hypoxia–check tracheal tube position, ventilate with 100% oxygen.
- Tension pneumothorax–check tracheal position, listen for air entry.
- Pacing failure – check pacing box output and pacing wire In asystole, secondary to a loss of cardiac pacing, chest compressions may be delayed momentarily as long as the surgically inserted temporary pacing wires can be connected rapidly and pacing re-established (DDD at 100 min−1 at maximum amplitude).
Defibrillation. There is concern that external chest compressions can cause sternal disruption or cardiac damage.457–460 In the post-cardiac surgery ICU, a witnessed and monitored VF/pVT cardiac arrest should be treated immediately with up to three quick successive (stacked) defibrillation attempts. Three failed shocks in the post-cardiac surgery setting should trigger the need for emergency resternotomy. Further defibrillation is attempted as indicated in the universal algorithm and should be performed with internal paddles at 20J if resternotomy has been performed.461,462
Emergency drugs.Use adrenaline very cautiously and titrate to effect (IV doses of up to 100mcg in adults). Consider amiodarone 300mg in patients with refractory shockable rhythms(VF/pVT), but do not delay resternotomy. Atropine is not recommended for asystole and temporary or external pacing should be employed.
Emergency resternotomy. This is an integral part of resuscitation after cardiac surgery, once all other reversible causes have been excluded. Once adequate airway and ventilation has been established, and if three attempts at defibrillation have failed inVF/pVT, undertake resternotomy without delay. Emergency resternotomy is also indicated in asystole or PEA, when other treatments have failed, and should be performed within 5 min of the cardiac arrest by anyone with appropriate training.
These guidelines are also appropriate for patients following non-sternotomy cardiac surgery, but surgeons performing these operations should have already made clear their instructions for chest reopening in an arrest.
Special considerations regarding treatment of patients with ventricular assist devices (VADs) are addressed in the section on special patients (see patients with ventricular assist devices).
Cardiac arrest in a cardiac catheterisation laboratory Cardiac arrest may occur during percutaneous coronary intervention(PCI) for ST-elevation myocardial infarction (STEMI) or non-STEMI, but it may also be a complication of an angiography such as catheter wedging, air or thrombus embolism in the coronary artery, coronary artery intim a dissection from the tip of the angiography catheter or caused by pericardial tamponade from a perforated coronary artery during the procedure. Most complications will resulting VF with immediate need for defibrillation. For this reason, patients must be continuously monitored and a defibrillator must be available in the angiography room. Self-adhesive radiolucent defibrillation pads may already be placed at the beginning of the procedure in high-risk patients.
In this special setting with immediate response to monitored VF, defibrillation without preceding chest compressions is recommended. As the patient is early in the electrical phase of a cardiac arrest, in contrast to the guidelines for unmonitored and OHCAs, the result of defibrillation (VF termination and ROSC) can be determined before chest compressions are started. If needed for failed defibrillation or immediately recurring VF, immediate defibrillation may be repeated up to two times.
If VF persists after the initial three shocks or ROSC not immediately established with certainty, chest compressions and ventilations must be initiated without further delay and a cause for the unresolved problem sought with further coronary angiography. It is of extreme importance that chest compressions are not interrupted for an giography. On an angiography table with the image intensifier above the patient, delivering chest compressions with adequate depth and rate is almost impossible and exposes the rescuers to dangerous radiation. Therefore, early transition to the use of a mechanical chest compression device is strongly recommended.247,463 If the problem is not rapidly resolved, very low quality evidence suggests that the use of extracorporeal life support (ECLS) can be considered as a rescue strategy if the infrastructure is available, and probably to be preferred over intra-aortic balloon pump (IABP).464 There is no evidence to recommend circulatory support with the Im pell a pump only during cardiac arrest. If the cardiac arrest is caused by a non-shock able rhythm, immediate transthoracic echocardiography should identify pericardial tamponade or other conditions.
Cardiac arrest in a dialysis unit
Introduction. Sudden cardiac death is the most common cause of death in haemodialys is patients and is usually preceded by ventricular arrhythmias.465 Hyperkalemia contributes to 2–5% of deaths amongst haemodialysis patients466 and accounts for up to 24% of emergency haemodialysis session in haemodialysis patients.467 The frequency of cardiac arrest is highest on the first session of haemodialysis of the week (i.e. Monday or Tuesday) as fluid and electrolyte disturbances peak after the weekend interval.468 Primary prevention of cardiac arrest in dialysis patients include the avoidance of low potassium dialysate solutions and proper use of medication, e.g. beta blockers or angiotensin-converting enzyme inhibitors.465 There is little evidence to guide the treatment of cardiac arrest during haemodialysis, although some special considerations have been suggested.469
Initial steps.
- Call the resuscitation and seek expert help immediately
- Follow the universal ALS algorithm
- Assign a trained dialysis nurse to operate the dialysis machine
- Stop ultrafiltration (i.e. fluid removal) and give afluid bolus.
- Return the patient’s blood volume and disconnect from the
- Leave dialysis access open and use for drug administration
- Beware of wet surfaces (i.e. dialysis machines may leak).
- Minimise delay in delivering defibrillation
Modifications to cardio pulmonary resuscitation.
Defibrillation. A shockable rhythm (VF/pVT) is more common in patients undergoing haemodialysis465,470,471 than in the general population.472,473 The safest method to deliver a shock during dialysis requires further study. Most haemodialysis machine manufacturers recommend disconnection from the dialysis equipment prior to defibrillation.474 Ensure familiarity with local dialysis equipment and check if equipment has defibrillator-prooflabel in accordance with the International Electrotechnical Committee (IEC) standards. Automated external defibrillators in nurse-led dialysis centres can facilitate early defibrillation by first responders with appropriate training.475
Vascular access. Use dialysis access in life-threatening situations and cardiac arrest.469
Potentially reversible causes.All of the standard reversible causes (4Hs and 4Ts) apply to dialysis patients. Electrolyte disorders, particularly hyperkalaemia (see hypo-/hyperkalaemia and other electrolyte disorders), and fluid overload (e.g. pulmonary oedema) are most common causes.
Post resuscitation care. Dialysis may be required in the early post resuscitation period guided by fluid status and serum biochemistry. Patient transfer to an area with dialysis facilities(i.e.intensivecare unitorrenal high dependency unit) is essential.
Cardiac arrest in the dental surgery
Introduction. Dental surgery emergencies include a variety of situations ranging from psychosomatic disorders precipitated by fear and anxiety to life-threatening situations requiring immediate lifesaving procedures. Cardiac arrest in primary dental practice is rare with an incidence of 0.002–0.011 cases reported perdentist per year.476–478
The most frequent medical emergencies include vasovagal (pre-) syncope, orthostatic hypotension, hypertensive crisis, hyperventilation, seizures, moderate allergic reactions, hypoglycaemia, and angina.476,479 The majority of dentists responded that they would be capable of performing initial treatment of common emergencies, while many felt unable to treat anaphylaxis, myocardial infarction, or cardiac arrest.476,477
A cardiac arrest occurring in a dental surgery is an event witnessed by medical professionals who have a duty of care and are required to be competent in the delivery of CPR.
Causes of cardiac arrest. Causes of cardiac arrest usually relate to pre-existing comorbidities or complications of the procedure. The life-threatening emergencies commonly arise from myocardial infarction, grand mal seizures or exacerbation of asthma. Dental procedures may cause loss of airway patency related to the primary pathology or complications of the procedure (e.g. bleeding, secretions, tissue swelling). Choking is rare, with are ported incidence of 0.07–0.09 cases per dentist per year.476,477 The addition of sedation is a contributory risk in these cases, although provision of dental treatment underboth local anaesthesia and sedation has an excellent safety record.480,481
Although life-threatening anaphylaxis is rare, it is a documented cause of death during dental procedures. In addition to chlorhexidine mouthwash, other common causes may include penicillin and latex. Anaphylaxis to local anaesthetics is very rare and a reaction to this class of drug is usually due to a direct intravascular injection of adrenaline contained in the solution. True anaphylaxis (all causes) occurs in only 0.004–0.013 cases perdentist peryear, compared with coronary symptoms (anginaor myocardial infarction) occurring in 0.15–0.18 cases per year.476,477
- Simultaneously open the airway and check breathing (look, listen, feel). If breathing is not normal or absent, assume a cardiac arrest until provenotherwise. Send someone to get an AED if available.
- Some case reports describe successful CPR in a patient left on a dental 482,483 Small simulation studies comparing the effectiveness of CPR on a dental chair and on the floor reported either lower or equivalent CPR quality.484–487 However, the patient should not be moved from the dental chair because of the risk of injury to the patient and rescuers and the limited space that is likely to be available on the floor next to the patient.482,483 Ensure that the dental chair is fully reclined into the horizontal position, support its head with as tool to increase stability, and start chest compressions immediately.482,484
- If feedback devices are used to monitor CPR quality, those using accelerometers may overestimate depth of compressions if used on a dental chair.488
- Follow the standard compression: ventilation ratios for adults and Consider the over-the-head technique of CPR if access to either side of the chest is limited.489–492
- Maintain the airway and ventilate the patient with a bag valve-mask device, using the two-hand technique if necessary. Supraglottic airways may be inserted if the operator is skilled in their use, but tracheal intubation is not a recommend
- Switch on the AED and follow the instructions. Deliver the first shock as soon as possible if indicated.
- Continue with CPR until signs of life return, or the patient’s handover to the professional resuscitation team (see adult basic life support and automated external defibrillation).493
Equipment and training. Follow national guidelines for recommended equipment to treat medical emergencies in a dental practice.478 Basic resuscitation equipment should be available immediately in all primary care dental premises, including suction, self-inflating bag with face masks, oxygen, and emergency drug kit.494,495 The role of early defibrillation should be emphasised to increase the availability of AEDs in dental surgeries,482,496 which is still unsatisfactory, ranging from a reported 0.5–2.6% in Europe497,498 to 11% in the United States.499 We recommend that all dental practices delivering clinical care have immediate access to an AED, with all staff trained in its use. Advanced equipment and special training is needed if analgesia or sedation is used in dental surgeries.478,500 In patients with pacemakers, ECG monitoring and immediate availability of a defibrillator is recommended if electrical devices are used (e.g. diathermy, electric pulptester, etc.).482
There is rightly a public expectation that dental practitioners and all other dental care professionals should be competent in treating cardio respiratory arrest.However, only 0.2–0.3% dentists have experience intreating a patient in cardiac arrest,476,479,501 and their training in CPR varies significantly between countries.476,477,501–503 Maintaining knowledge and competence to deal with medical emergencies must be an important part of the training of dentists. All dental care professionals should undergo annual practical training in the recognition and management of medical emergencies, and the delivery of CPR, including basic airway management and the use of an AED.478
Cardiac arrest in transportation vehicles
In-flight emergencies aboard airplanes
Introduction. Worldwide, 3.2 billion passengers flyon commercial airlines annually. The incidence of in-flight medical emergencies has been reported to be one event per 10,000–40,000 passengers.504,505 The probability of at least one medical incident reaches 95% after 24 intercontinental flights.505 Most of the cases involve middle-aged people.506 Two large studies recently reviewed more than 22,000 in-flight emergencies from five American and two European airlines. The most common medical problems were syncope or presyncope (37.4–53.5%), respiratory symptoms (12.1%), gastrointestinal problems(8.9–9.5%), and cardiac conditions (4.9–7.7%) with some variations across airlines.504,507 Surgical problems (e.g. deep venous thrombosis, appendicit is, gastrointestinal bleeding) were seen rarely(<0.5%).504
In-flightincapacitationoftheflightcrewisveryrare,themost common cause being acute myocardial ischaemia.508
The in-flight medical emergencies have very limited access to medical care, but the majority can be managed conservatively with fluids, oxygen and other treatment available from first aid kits on board. However, a quarter of these patients subsequently require additional evaluation in a hospital.507 Immediate diversion of an aircraft is requested in 2.4–7.3% of all incidents, most commonly due to chest pain, suspected stroke, and seizures.504,507,509,510
Cardiac arrest onboard has an incidence of 1 per 5–10 million passenger flights. An initial shockable rhythm is present in 25–31% patients,505,511–513 and the in-flight use of an AED can result in 33–50% survival to hospital discharge.511,513,514 Factors contributing to a high survival rate include a witness edevent, cabin crew trained in BLSandin 73–86% of cases, travelling medical professionals also providing immediate assistance.504,507,509 However, approximately 1000 lives are lost per year in International Airlines Transport Association(IATA) carriers. Some studies have shown that41–59% of cardiac arrests onboard are unwitnessed, occurring during sleep. There were no survivors if the initial rhythm was asystole or an idioventricular rhythm.511,513
Cardiopulmonary resuscitation on the airplane. In case of cardiac arrest, follow the universal algorithm for BLS (see adult basic life support and automated external defibrillation).493 Immediately request an AED and a first aid kit from cabin crew. Physicians and trained medical providers, e.g. nurses or EMS personnel, should also ask for advanced medical equipment. According to competencies and equipment available, provide the patient with advanced treatment, assuring that there is high quality CPR ongoing, an danAED was deployed appropriately (see adult advanced life support).168
Consider the following modifications to CPR:
- Introduce yourself to the cabin crew and state your professional qualifications.
- In case of cardiac arrest, performance of CPR is limited in an aircraft aisle due to Immediatelytransferthe patienttoasuitablelocation,e.g.galley or exit area. Consider an over-the-head technique of CPR if access precludes conventional CPR.489–492
- During CPR, attach oxygen to the face mask or self-inflating bag.
- Request immediate flight diversion to the nearest appropriate In other non-critical medical emergencies, coordinate an optimal course of action with the flight crew. Considerations for flight diversion will depend on the patient’s condition and on the need for immediate treatment in a hospital: e.g. acute coronary syndrome, stroke, persistently altered mental status; but also technical and operational factors.
- Ask cabin crew whether medical consultation is provided by the airline, g. radiotelephony or satellite communication.506,510
- An AED with a monitor can be safely attached to a non-arrested patient for monitoring heart rhythm, e.g. syncope, chestpain, or507, 512, 513
- Concerns about legal responsibility may arise when travelling physicians are asked Based on ethical duties, every physician is required to offer help within his or her scope of practice, but the legal duty is only applicable for certain countries. However, the so-called Good Samaritan Actandother regulations, depending on the origin of an aircraft, always protect healthcare providers helping on board from possible legal consequences.504,515
- Death on board can legally be confirmed only by a physician. If a dead person is found, or CPR has been terminated (see ethics of resuscitation and end-of-life decisions),243 flight diversion is not recommended.
Education and equipment.
Flight crew training. Both pilots and cabin crew mus treceive initial and recurrent training on emergency medical event procedures and operation of emergency medical equipment, including AEDs and first aid kits, but local operational procedures may also apply.516
Although civil aviation is regulated by a variety of national and internationallaws, some studies imply that the majority of in-flight emergencies stay unreported or are reported inconsistently.504, 517
Documentation of in-flight emergencies needs standardisation in order to improve cabin crew training and pre-flight assessments of selected groups of passengers.
On-board emergency equipment. The Federal Aviation Administration (FAA) requires every US registered commercial aircraft with a maximum pay load capacity of more than 7500 pounds and with at least one flight attendant to carry an AED, intravenous drugs, and advanced emergency equipment,518 while related regulations in Europe are less precise.519 On every commercial aircraft registered in Europe, there must be a first-aid kit that all cabin crew members are trained to use. Aircraft with at least 30 seats must also carry an advanced medical kit, which can be used by competent personnel, although the contents vary significantly and may be inadequate for all but the most basic of emergencies.504,517,520 Although most large European airlines carry AEDs, some of them only do so for inter continental flights, but some do not even provide any equipment for CPR.517
Based on the outcome data from survivors of cardiac arrest and in the absence of any alternative treatment for shockable rhythms on board, we strongly recommend mandatory AEDs in all commercial European aircraft, including regional and low- cost carriers.
Healthcare professionals should be aware of the on-board medical equipment and commonly encountered medical conditions in order to provide appropriate emergency treatment on request.505 Distribution of supportive information to travelling physicians should be encouraged, e.g. ‘Doctor on Board’ programme introduced by Lufthansa and Austrian Airlines in 2006.
Cardiac arrest in HEMS and air ambulances
Introduction. Air ambulance services operate either a helicopter emergency medical service (HEMS) or fixed-wing air ambulances that routinely transport critically ill patients directly to specialty centres and perform secondary transfers between hospitals. Cardiac arrest may occur in flight, both in patients being transported from an accident site and also critically ill patients being transported between hospital.521,522 In a retrospective analysis of 12,140 aero medical journies, the incidence of cardiac arrest in flight was low(1.1%). Fourty-three percent were medical patients and 57% were patients with traumatic injuries. In the medical cohort, the rate of ROSC was 75%.523
The extent of treatment available onboard of an air ambulance varies and depends on medical and technical factors, e.g. crew competences and configuration, cabin size and equipment. Ideally, all interventions should be performed before flight so that the need for unplanned treatment during flight is avoided.
Pre-flight preparation.When preparing transport of a critically ill patient, ensure that all necessary monitoring is attached and functioning. Check that IV access is secured and easily accessible and that all necessary drugs and medical equipment are available during flight.
Diagnosis. In monitored patients, asystole and shockable rhythms (VF/pVT) can be immediately identified, but recognition of PEA may be challenging, especially under sedation or general anaesthesia. Unexpected loss of consciousness (in alert patients), change of ECG pattern, and loss of the pulse oximeter signal should provoke a pulse and patient check. A sudden decrease in ETCO2 values in those being ventilated or loss of a waveform in those breathing spontaneously with ETCO2 monitoring are also indicators of cardiac arrest.
Treatment. Cardiac arrest in the air ambulance services should be treated according to the universal ALS algorithm. Start chest compressions and ventilation immediately after confirmation of cardiac arrest, attach monitoring (if not already), and follow universalALS algorithm.168 If a shockable rhythm (VF/pVT) is recognised in a monitored patient and defibrillation can be accomplished rapidly, immediately give up to three-stacked shocks before starting chest compressions. In a US study, 33% of patients achieving ROSC following defibrillation did not require any chest compressions.523
In smaller helicopters, there may be insufficient room to perform effective resuscitation and an emergency landing may be necessary to allow better patient access. Mechanical chest compression devices enable delivery of high quality chest compressions in the confined space of an airambulance and their use should be considered.248,524 If a cardiac arrest during flight is thought to be a possibility, consider fitting the patient within a mechanicalchestcompressiondeviceduringpackaging before flight.50,525
Cardiac arrest during sports activities Resuscitation on the field of play
Introduction. The sudden and unexpected collapse, not associated with contact or trauma, of an athlete on the field of play is probably cardiac in origin and requires rapid recognition and effective treatment if the victim is to survive. Sudden cardiac death (SCD) is the most common cause of death of athletes during competition and training. Estimates of the incidence of SCD vary according to the methodology but recently the incidence has been quoted as 1:11,394 in basketball players, 1:21,293 for swimmers and 1:41,695 for cross-county athletes with a wide variation between male and female athletes (incidence expressed as number of athletes per year).526 Hypertrophic cardiomyopathy (HCM) and arrhythmogenic right ventricular cardiomyopathy (ARVC) are the most common causes in under 35 year olds whilst at herosclerotic coronary artery disease accounts for 80% of sudden cardiac arrests in over 35 year olds.527 Congenital coronary artery abnormalities have been reported in 12–33% of athletes.528
Commotiocord is, the disruption of cardiac rhythm by a blow to the precordium, has a quoted incidence of 3%.529 The striking object must strike the chest within the cardiac silhouette within a 20 ms window of the upstroke of the T-wave.530 The overall survival rate from commotiocordis is reported to have improved with survival rates of up to 58% reported in recent years.531
Whatever the cause the sudden collapse of an athlete there should be an immediate response from the officials or medical team. The standard resuscitation procedures must be followed but with certain additional considerations as described below.
Access. The medical team should gain immediate access to the field of play. It is important that the medical team do observe access rules to the field of play but it would be hoped that the field of play officials will recognise or be alerted to the collapsed athlete and halt play so that it is safe to approach the competitor.
Where there is no medical team, during informal competition or in practice it is the responsibility of the referee, the coach or of the athletes’ colleagues to recognise the collapse and to initiate a call for help and resuscitation.
Calling for help. The call for help is essential to providing the collapsed athlete with the best chance of survival. It is essential that sports officials, coaches and sports organisers have a plan for medical collapse or trauma. In its simplest form this could include ensuring the availability of a mobile telephone and knowledge of the site/ (field of play, clubhouse) to provide best access for the ambulance. It would be hoped that more officials and coaches will be trained in BLS and AED usage.
Resuscitation. If the athlete is unresponsive and not breathing normally, commence BLS. If available attach an AED and follow the instructions; if this is SCD then the rhythm will probably be ventricular fibrillation and will respond to defibrillation.
The sports field of play is often an openarenaand in major competition may be on view to many thousands of spectators and a television audience. Although treatment must not be compromised moving the collapsed athlete to a quieter and more private site for continued treatment may be considered. Where there is not an immediate response to treatment and there is an organised medical team, this move could be accomplished after three defibrillation attempts on the rationale of providing the highest efficacy of defibrillation in the first three shocks. The move, if decided, should be agreed and may need to be accomplished in stages to allow for near continuous chest compressions. Where there is no medical team or a defibrillator is not immediately available then BLS must continue until advanced care arrives.
If the athlete responds to resuscitation then they must be transported immediately to the nearest cardiac centre for further evaluation and treatment. As there is a possibility of the rhythm reverting this transportation must be under the supervision of a healthcare professional who is equipped and capable of administering resuscitation and further defibrillation.
Prevention. In an effort to predict and prevent SCD, the International Olympic Committee Medical Commission (IOC Medical Commission 2014) and many International Sport Federations have recommended cardiac screening for athletes. However, there is much debate about the effectiveness of the techniques being used and the population that should be screened.532
Water rescue and drowning
Introduction
Drowning is a common cause of accidental death.533 Prompt and effective actions by bystanders, trained rescuers and emergency medical personnel can make the difference between life and death.534–536 These guidelines provide advice about the initial rescue and resuscitation of victims involved in drowning incidents. They are intended for healthcare professionals and certain groups of lay responders that have a special responsibility in the care of the drowning victim, e.g. lifeguards, life boat crews, swimmingpool instructors and water rescueteams.
Epidemiology
The World Health Organization (WHO) reports that every hour of everyday, more than 40 people lose their lives to drowning; 372,000 deaths each year.537 The WHO acknowledges that the true number of drownings worldwide is much higher. More than 90%of these deaths occur in low and middle-income countries. The incidence of drowning varies between countries, witheasternEurope having the highest rates in Europe.533 Although risk groups vary between countries, in general males are much more likely to drown than females.Most accidental drownings are children who are unable to swim.In countries where aquatic leisure in combination with alcohol and drug use is common, young adults are a second group of risk.538,539 Many countries also report a slight increase of drowning in the age groups above 70 years related to accidents and physical activities close to water. Drowning is commonest in inland waters (e.g. lakes, rivers) and during summer months.538–540
Definitions, classifications and reporting
The International Liaison Committee on Resuscitation (ILCOR) defines drowning as a process resulting in primary respiratory impairment from submersion/immersion in a liquid medium. Implicit in this definition is that a liquid/air interface is present at the entrance of the victim’s airway, preventing the victim from breathing air. The victim may live or die after this process, but whatever the outcome, he or she has been involved in a drowning incident.541 Submersion occurs when the face is underwater or covered in water. Asphyxia and cardiac arrest occurs within a matter of minutes of submersion. Immersion, by contrast, is when the head remains above water, in most cases by means of the support of a lifejacket. In most situations of immersion, the victim remains immersed with an open airway and becomes hypothermic, although aspiration of water may occur if water splashes over the face or if the victim becomes unconscious with their face in the water. The difference between submersion and immersion is important in understanding the difference in epidemiology, pathophysiology, clinical course and prognostic parameters between the two drowning processes.
If the casualty is rescued, the process of drowning is interrupted, which is termed a non-fatal drowning. If the person dies at any time as a result of drowning, the term is fatal drowning. Avoid terms such as dry and wet drowning, active and passive drowning, silent drowning, secondary drowning and near-drowning.541 To improve consistency in information between studies use the Utstein-style registration template for drowning when reporting outcomes from drowning incidents.542
Pathophysiology
Detailed summaries of the pathophysiology of drowning have been published.536,541,543,544 In brief, following submersion, the victim initially breath holds by reflex. During this time the victim frequently swallows water. As breath holding continues, hypoxia and hypercapnia develop. A reflex laryngospasm may temporarily prevent the entrance of water into the lungs. Eventually these reflexes abate and the victim aspirates water. The key feature to note in the pathophysiology of drowning is that brady cardia as a consequence of hypoxia occurs before sustaining a cardiac arrest. Correction of hypoxaemia by ventilation only resuscitation is critical and in itself may lead tore turn of spontaneous ventilation or circulation(ROSC) in some cases, probably because the presence of a circulation had not been detected.545–549
Drowning chain of survival
The Drowning Chain of Survival describes five critical links for improving survival from drowning (Fig. 4.5).535 The first two links cover prevention of drowning and recognition of distress.550,551
This chapter provides guidance on removal from water, initial and post resuscitation care.
Water rescue
Bystander response. Bystanders play a critical role in initial attempts at rescue and resuscitation.534,548,552–555 At the same time, bystanders who attempt a rescue have died during the rescue attempt, mostly when drowning occurs in surf or fast moving water.556 Whenever possible, bystanders should attempt to save the drowning victim without entry into thewater. Talking to the victim, reaching with a rescue aid (e.g. stick or clothing), or throwing a rope or buoyantrescueaidmay be effective if the victim is close to dryland. If entry into the water is essential, take a buoyant rescue aid, flotation device or boat.535 It is safer to enter the water with two rescuers than alone. Never dive head first in the water when attempting a rescue. You may lose visual contact with the victim and run the risk of a spinal injury.
Trained rescuer response. Trained rescuers are often professionals who work in teams with specialist equipment to assist with search and rescue. Where the rescue takes time, the teams often seek guidance on the likelihood of survival. For this reason, ILCOR reviewed specific prognostic indicators and noted that submersion durations of less than 10 min were associated with a very high chance of favourable outcome; submersion durations longer than 25 min were associated with a low chance of favourable outcomes.557
Age, emergency medical services (EMS) response time, fresh or salt water, water temperature, and witness status were not useful for predicting survival. Submersion in ice-cold water may prolong the window of survival and justify extended search and rescue activities.558–560
In-water resuscitation. Trained individuals may undertake in water ventilation ideally with the support o fa buoyant rescue aid.545,561,562 If a rescuer, in general a surf-lifeguard, finds a non-responding drowning victim in deep open water, the rescuer may start ventilation when trained to do so before moving the victim to dryland or rescue craft. Some victims may respond to this.If not responding, and depending on the local situation, such as sea conditions, distance to shore, availability of rescue boat or rescue helicopter, the rescuer should then decide to bring the victim to shore as quickly as possible without further ventilation while rescue-swimming with the victim or continue on the spot with in water ventilation until support by crews of a rescue boat or rescue helicopter arrives to take over there suscitation. One study suggests that the second option has a higher survival rate.545
Fig.4.5. Drowning chain of survival.535
Reproduced with permission from Elsevier Ireland Ltd.
Removal from water. Remove the victim from the water promptly. The chances of a drowning victim sustaining a spinal injury are very low.563 Spinal precautions are unnecessary unless there is a history of diving in shallow water, or signs of severe injury after water-slide use, waterskiing, kite-surfing, or watercraft racing.If the victim is pulseless and apnoeic, remove them from the water as quickly as possible while attempting to limit neck flexion and extension. Hypovolaemia after prolonged immersion may cause a circum-rescue collapse/arrest. Keep the victim in a horizontal position during and after retrieval from the water.
Initial resuscitation once retrieved from water Follow the standard BLS sequence, initially by checking for response, opening the airway and checking for signs of life. The drowning victim rescued from the water within a few minutes of submersion is likely to exhibit abnormal (agonal) breathing. Do not confuse this with normal breathing.
Rescue breaths/ventilations. The BLS sequence in drowning (Fig. 4.6) reflects the critical importance of rapid alleviation of hypoxia. Inflation should take about 1 sand be sufficient to see the chest rise. However it often takes more time to insuflate air than under normal conditions due to reduced compliance and high airway resistance. The higher inflation pressure may precipitate inflation of the stomach with regurgitation and also reduce cardiac output. Expert opinion suggests that cricoid pressure applied by trained and skilled personnel in casualties without a secured airway may reduce gastric inflation and enhance ventilation in drowning.
Fig.4.6. Drowning treatment algorithm for rescuers with a duty to respond.
Chest compressions. If the victim has not responded to initial ventilations, they should be placed on a firm surface before starting chest compressions, as compressions are ineffective in the water.564,565
Provide CPR in a ratio of 30 compressions to 2 ventilations. Most drowning victims will have sustained cardiac arrest secondary to hypoxia. In these patients, compression-only CPR is likely to be ineffective and should be avoided.
If sufficient rescuers are present, the person performing the aquatic rescue should be relieved of continuing CPR once on land as they are likely to be fatigued, which may impair the quality of CPR.566, 567
Automated external defibrillation. Defer using an AED until after CPR has commenced. Dry the victim’s chest, attach the AED pads and turn the AED on. Deliver shocks according to the AED prompts.
Fluid in the airway. In some situations, massive amounts of foam caused by admixing moving air with water are seen coming out of the mouth of the victim. Do not try and attempt to remove the foam as it will keep coming. Continue rescue breaths/ventilation until an ALS provider arrives and is able to intubate the victim. Regurgitation of stomach contents and swallowed water is common during resuscitation from drowning.568 If this prevents ventilation completely, turn the victim on their side and remove the regurgitated material using directed suction if possible.
Modifications to advanced lifesupport
Airway and breathing. During the initial assessment of the spontaneously breathing drowning victim, give high-flow oxygen (10–15Lmin−1), ideally through an oxygen mask with reservoir bag.127 For victims who fail to respond to these initial measures, who have a reduced. level of. consciousness or are in cardiac arrest, consider early tracheal intubation and controlled ventilation by skilled personnel. Reduced pulmonary compliance requiring high inflation pressures may limit the use of a supraglottic airway device.569 Take care to ensure optimal preoxygenation before attempting tracheal intubation. Pulmonary oedema fluid may pour from the airway and may need continuous suctioning to enable a view of the larynx. After the position of the tracheal tube is confirmed, titrate the inspired oxygen concentration to achieve a SpO2 of 94–98%.127 Pulse oximetry can give spurious readings following rescue from drowning.570
Confirm adequate oxygenation and ventilation with arterial blood gases once available. Set positive end expiratory pressure (PEEP) to at least 5–10 cm H2O. However, PEEP levels of 15–20 cm H2O may be required if the patient is severely hypoxaemic.571 Decompress the stomach with a gastric tube.
Circulation and defibrillation. Palpation of the pulse as the sole indicator of the presence or absence of cardiac arrest is not always reliable. As soon as possible, use information from monitoring modalities such as the ECG trace, ETCO2 and echo cardiography to confirm the diagnosis of cardiac arrest.
If the victim is in cardiac arrest, follow standard ALS protocols. If the victim is hypothermic, modify the approach in accordance with the guidance for treatment of hypothermia (see hypo-/hyperthermia).
After prolonged immersion, most victims will have become hypovolaemic due to the cessation of the hydrostatic pressure of water on the body. Give rapid IV fluid to correct hypovolaemia. This should commence out-of-hospital if transfer time is prolonged.
Discontinuing resuscitation efforts
Making a decision to discontinue resuscitation efforts on a victim of drowning is notoriously difficult. No single factor can accurately predict good or poor survival with certainty. Frequently, decisions made in the field later prove to have been incorrect.572
Continue resuscitation unless there is clear evidence that such attempts are futile (e.g. massive traumatic injuries, rigourmortis, put refaction, etc.), or timely evacuation to a medical facility is not possible. Neurologically intact survival has been reported in several victims submerged for longer than 25 min, however these rare case reports almost invariably occur in children submerged in ice-cold water, when immersion hypothermia has preceeded hypoxia or in submersion of car occupants.558,559,573,574 A retrospective study of 160 children who drowned in the Netherlands found that outcomes were extremely poor if ALS took longer than 30 min to achieve ROSC even if hypothermia was present.560
Post resuscitation care
Salt versus fresh water. Small differences in electroly tedisturbance are rarely of any clinical relevance and do not usually require treatment.575,576
Lung injury. The predominant pathophysiological process in the lungs is driven by surfactant wash-out and dysfunction, alveolar collapse, atelectasis, and intrapulmonary shunting. The severity of lung injury varies from a mild self-limiting illness to refractory hypoxaemia. Many victims of drowning are at risk of developing acute respiratory distress syndrome (ARDS).577 Although there are no randomised controlled trials undertaken specifically in this population of patients, it seems reasonable to include strategies such as protective ventilation that have been shown to improve survival in patients with ARDS.578,579 Extracorporeal membrane oxygenation(ECMO) has been used for those in refractory cardiac arrest, those with refactory hypoxaemia and in selected cases of submersion in icecold water, although success rates remain low.580–583 Pneumonia is common after drowning. Prophylactic antibiotics have not been shown to be of benefit but they may be considered after submersion in grossly contaminated water such as sewage. Give broad-spectrum antibiotics if signs of infection develop subsequently.583–587
Neurological outcome. Neurological outcome, notably severe permanent neurological damage, is primarily determined by the duration of hypoxia. Attempts have been made to improve neurological outcome following drowning with the use of barbiturates, intracranial pressure (ICP) monitoring, and steroids. None of these interventions has altered outcome.588
Wilderness and environmental emergencies
Difficult terrain and remote areas
Geographical and meteorological considerations. Compared to urban areas some terrains will be more difficult to access and are remote from organised medical care. Exposed and steep terrain may render extrication dangerous and challenging. The chances of a good outcome from cardiac arrest may be reduced because of delayed access and prolonged transport. Furthermore, some environments are harsher than urbanareas(e.g. cold, windy, wet, very bright due to light-reflection on ice and snow). Human and material resources may be greatly restricted.589,590
Compared with the partial pressure of oxygen at sea level (PO2 about 21 kPa/159 mmHg), the PO2 at high (>1500 m above sea level), very high (3500–5500 m) and extreme altitude (>5500 m) will be progressively lower, constraining the physical activity of rescuers. There is a physiological limit to acclimatisation (e.g. short term– hyperventilation and increased cardiac output; longterm–haemoglobin increase). The highest permanent settlement is at 5100m (PO2 about 11k Pa/84 mm Hg). Above 7500m the risk of lethal acute altitude illness is very high.
There are no epidemiologicaldata on the causes of cardiac arrest at high altitude. However, it is conceivable that primary cardiac arrest is the major(60–70%) cause of sudden cardiac arrest. Thus, public access defibrillator (PAD) programmes in populated areas at altitude seem reasonable. For instance, public access defibrillators (PADs) should be placed in popular ski areas, busy mountain huts and restaurants, at mass-participation events, and in remote but often-visited locations that are not medically covered.591 In areas where physicians are regularly involved in mountain rescue operations, the provided on-site treatment is more inline with resuscitation guidelines.592
Decision making. Continuous monitoring and treatment may be difficult during transport because the patient will be insulated from the harsh environment within a rescue bag, being well wrapped and secure donastretcher. During transport, CPR may be limited inquality and nearly impossible in some circumstances (e.g.while carrying the patient, during abseiling or winching). In dangerous and difficult terrain where continuous CPR is impossible, delayed and intermittent CPR has been proposed for hypothermic patients.45 Mechanical resuscitation devices may help to improve CPR quality during difficult extrication and prolonged transport.50
Transportation
Effective and safe immobilisation and splinting will reduce morbidity and mortality.593 Whenever possible, transport the patient with air rescue.593, 594 The organisation of the helicopter emergency medical service (HEMS) affects the outcome.
High altitude illness
Given the increasing popularity of travel at altitude, an increasing number of tourists at altitude have cardiovascular and metabolic risk factors for cardiac arrest. The pO2 falls with increasing altitude and this oxygen deficiency may lead to acute manifestations of mountain sickness.
Persons travelling to an altitude of >3500m are at risk of developing:
- acute mountain sickness (AMS) with headache, nausea, fatigue and dizziness;
- high altitude pulmonary oedema (HAPO) with severe dyspneoa and cyanosis;
- high altitude cerebral oedema (HACO) with gate disorder, disorientation and confusion.
Risk factors include a fast rate of ascent and a previous history of mountain sickness. If not treated promptly, HAPO and HACO may progress rapidly to loss of consciousness, severe respiratory distress, circulatory instability and cardiac arrest. The most important actions are immediate descent or transport to lower levels of altitude, administration of oxygen(2–6L min−1, target>90%SpO ), treatment in a portable hyperbaric chamber, in cases of HACO administration of dexamethasone 4–8 mg every 8h, and in cases of HAPO, nifedipine 30mg every 12h.
Resuscitation at high altitude does not differ from standard CPR. With the lower pO2, CPR is more exhausting for there scuer than at sea level, and the average number of effective chest compressions may decrease within the first minute.598–600 Use mechanical chest compression devices whenever possible.
Commonly, no physician will be present to give guidance to nurses or paramedics on when to stop CPR. Guidelines have therefore been proposed for these situations.46
CPR may be with held or terminated in a patient with absent vital signs when:
- the risk is unacceptable to the rescuer
- the rescuer is exhausted
- extreme environments prevent CPR
- any of the following apply:
- decapitation
- truncalt ran section
- whole body incine rated
- decomposed
- frozen solid
- avalanche victim in asystole with obstructed airway and burial time > 60 min(see avalanche burial below).
- CPR may be also terminated when all of the following criteria apply:
- unwitnessed loss of vital signs;
- no ROSC during 20 min of CPR;
- no shock advised at anytime by AED or only asystole on ECG;
- no hypothermia or other reversible causes warranting extended CPR.
In situations where transport is not possible, and correction of reversible causes is not possible, further resuscitation is futile and CPR should be terminated. These recommendations should be interpreted in the context of local conditions and legislation.
Avalanche burial
Introduction. In Europe and North America together, there are about 150 snow avalanche deaths each year. Most are sports related and involve skiers, snowboarders and snowmobilers. Fatalities are mainly due to a sphyxia, sometimes associated with trauma and hypothermia. Prognostic factors are severity of injury, duration of complete burial, airway patency, core temperature and serum potassium.601 Completely buried avalanche victims die from asphyxia within 35 min if the airway is obstructed. The average cooling rate is 3◦Ch−1,602 ranging from 0.6◦Ch−1 to 9◦Ch−1603, 604; moderate to severe hypothermia may become important after 60 min of burial if the airway is patent. The highest recorded potassium in an avalanche victim who was successfully resuscitated is 6.4 mmol L−1.601,605–607 The survival rate of avalanche victims presenting with cardiac arrest ranges from 7% to 17%.605,606 Survival patterns differ across countries due to terrain, climate and prehospital medical care.56, 608, 610
Decision-making on scene. Avalanche victims are not likely to survive when they are:
- buried>60min(or if the initial core temperature is<30◦C) and in cardiac arrest with an obstructed airway on extrication;
- buried and in cardiac arrest on extrication with an initial serum potassium>8m molL−1.
Full resuscitative measures, including extracorporeal rewarming, are indicated for all other avalanche victims without evidence of an unsurvivable injury.
Avalanches occur in areas that are difficult to access by rescuers in a timely manner, and burials frequently involve multiple victims. The decision to initiate full resuscitative measures should be determined by the number of victims and the resources available, and should be informed by the likelihood of survival.601 As adherence to present guidelines is poor,611,612 the use of a standardised checklist is recommended.613
Management of completely buried avalanche victims. The algorithm for the management of buried avalanche victims is shown in Fig.4.7:
- In all cases, extricate the body gently and use spinal precautions.
- Consider withholding resuscitation at the scene if it increases risk to the rescue team or if the victim is lethally injured or completely frozen.
- If unknown, core temperature may substitute for decision-making.
- If the duration of burial is ≤60 min (or initial core temperature is ≥30◦C) and cardiac arrest is confirmed, follow standard ALS guidelines (see adult advanced life support).168 During CPR, measure core temperature, monitor ECG, give oxygen and apply insulation and heat packs to the trunk. Give drugs and fluids only if IV or IO access can be established within a few minutes. Resuscitation may be terminated in a norm other micasystolic patient if ALS is not successful after 20 min, inanabsence of reversible cause (see ethics of resuscitation and end-of-life decisions).243
- Transport survivors with concern of respiratory (e.g. pulmonary oedema) or other- system critical illness or injury to the most appropriate medical centre. Provide specific trauma care as indicated. The admitting hospital must be capable of advanced active
- If the duration of burial is>60min (or initial core temperature is<30◦C) and cardiac arrest is confirmed, start CPR and attach If there is any electrical activity or a patent airway in anasystolic patient, continue CPR. Defibrillation beyond three attempts maybe delayed until core temperature ≥ 30◦C.
- Transport all patients who present with cardio vascular instability (i.e. ventricular arrhythmias, systolic blood pressure< 90 mmHg) or core temperature< 28◦C to an ECLS rewarming centre. Follow regional hypothermia protocols if needed.
- If direct transport to an ECLS rewarming centre is not possible in a timely manner, e.g. by HEMS, check potassium level at the nearest. If potassium exceeds>8m molL−1, consider terminating resuscitation (after excluding crush injuries and considering if depolarizing muscle relaxants were used).
Lightning strike and electrical injuries
Introduction. Electrical injury is a relatively infrequent but potentially devastating multi system injury with high morbidity and mortality, causing 0.54 deaths per 100,000 people each year. Most electrical injuries occur indoors. In adults, electrical injuries are common in the workplace and are generally associated with high voltage, whereas children are at risk primarily at home, where the voltage is lower (220V in Europe, Australia and Asia;110V in the United States and Canada).614 Electrocution from lightning strikes is rare, but worldwide it causes 1000 deaths each year.615
Electric shock injuries are caused by the direct effects of current on cell membranes and on vascular smooth muscle. The thermal energy associated with high-voltage electrocution will also cause burns. Factors influencing the severity of electrical injury include whether the current is alternating (AC) or direct (DC), voltage, magnitude of energy delivered, resistance to current flow, pathway of current through the patient, and the area and duration of contact. Skin resistance is decreased by moisture, which increases the likelihood of injury. Electric current follows the path of least resistance; conductive neuro vascular bundles within limbs are particularly prone to damage. Contact with AC may cause tetanic contraction of skeletal muscle, which may prevent release from the source of electricity. Myocardial or respiratory failure may cause immediate death:
- Respiratory arrest may be caused by paralysis of the central respiratory control
- Current may precipitate ventricular fibrillation (VF) if it traverses the myocardium during the vulnerable period (analogous to an R-on-T phenomenon).616 Electrical current may also cause
Fig. 4.7. Avalanche accident algorithm. Management of completely buried victims.(ECLS, extra corporeal lifesupport).
myocardial ischaemia because of coronary artery spasm. Asystole may be primary, or secondary to as phyxia following respiratory arrest.
Current that traverses the myocardium is more likely to be fatal. Atransthoracic (hand-to-hand) pathway is more likely to be fatal than a vertical (hand-to-foot) or straddle(foot-to-foot)pathway. There may be extensive tissue destruction along the current pathway.
Associated injuries are common. Blast (hyperbaric) injuries, injuries from being. thrown from the point of contact and tetanic contraction causing limb fractures have all beenreported.
Lightning strike. Lightning strikes deliver as much as 300 kV over a few milli seconds. Most of the current from a lightning strike passes over the surface of the body in a process called‘external flashover’. Both industrial shocks and lightning strikes cause deep burns at the point of contact. For industrial shocks the points of contact are usually on the upper limbs, hands and wrists, whereas for lightning they are mostly on the head, neck and shoulders. Injury may also occur indirectly through ground current or current splashing from a tree or other object that is hit by lightning.617 Explosive force may cause blunt trauma.618 The pattern and severity of injury from a lightning strike varies considerably, even among affected individuals from a single group.619–621 As with industrial and domestic electric shock, death is caused by cardiac620–624 or respiratory arrest.617,625 In those who survive the initial shock, extensive cate-cholamine release or autonomic stimulation may occur, causing hypertension, tachycardia, non-specific ECG changes (including prolongation of the QT interval and transient T-wave inversion), and myocardial necros is. Creatinekinase may be released from myocardial and skeletal muscle. Lightning canal so cause central and peripheral nerve damage; brain haemorrhage and oedema, and peripheral nerve injury are common. Mortality from lightning injuries is as high as 30%, with up to 70% of survivors sustaining significant morbidity.626,627
Diagnosis. The circumstances surrounding the incident are not always known. Unique pattern of skin lesions called feathering or Lichtenberg figure is a pathognomonic symptom that is seen only in patients struck by lightning.628 Unconscious patients with linear or punctuate burns (feathering) should be treated as victims of lightning strike.617
Safety measures. Ensure that any power source is switched off and do not approach the casualty until it is safe.High-voltage (above domestic mains) electricity can arc and conduct through the ground for up to a few metres around the casualty. It is safe to approach and handle casual ties after lightning strike, although it would be wise to move to a safer environment, particularly if lightning has been seen within 30min.617
Resuscitation. Patients struck by lightning are most likely to die if they sustain immediate cardiac or respiratory arrest and are not treated rapidly. Whenmultiple victimsarestrucksimultaneously by lightning, rescuers should give highest priority to patients in respiratory or cardiac arrest. Victims with respiratory arrest may require only ventilation to avoid secondary hypoxic cardiac arrest. Resuscitative attempts may have higher success rates in lightning victims than in patients with cardiac arrest from other causes, and efforts may be effective even when the interval before there suscitative attempt is prolonged.625 Dilated or non-reactive pupils should never be used as a prognostic sign, particularly in patients suffering a lightning strike.617
Start standard BLS and ALS without delay:
- Airway management may be difficult if there are electrical burns around the face and neck. Early tracheal intubation is needed in these cases, as extensive soft-tissue oedema may develop causing airway obstruction. Head and spine trauma can occur after Immobilise the spine until evaluation can be performed.
- Muscular paralysis, especially after high voltage, may persist for several hours627; ventilatory support is required during this period.
- VF is the commonest initial arrhythmia after high-voltage AC shock; treat with Asystole is more common after DC shock; use standard protocols for this and other arrhythmias.
- Vigorous fluid therapy is required if there is significant tissue destruction. Maintain a good urine output to enhance the excretion of myoglobin, potassium and other product soft issue 624
- Maintain spinal immobilisation if there is a likelihood of head or neck629, 630
- Conduct a thorough secondary survey to exclude traumatic injuries caused by tetanic muscular contraction or by the person being thrown.629,631
- Electrocution can cause severe, deep soft-tissue injury with relatively minor skin wounds, because current tends to follow neuro vascular bundles; look carefully for features of compartment syndrome, which will necessitate fasciotomy
- Although rare, consider abdominal visceral injuries caused directly by electrical
There are conflicting reports on the vulnerability of the fet us to electric shock. The clinical spectrum of electrical injury ranges from a transient unpleasant sensation for the mother with no effect on her fetus, to placental abruption, fetalburn or intrauterine fetal death either immediately or a few days later. Several factors, such as the magnitude of the current and the duration of contact, are thought to affect outcome.632
Further treatment and prognosis. Immediate resuscitation of young victims in cardiac arrest from electrocution can result in long-term survival. Successful resuscitation has been reported after prolonged lifesupport.
All those who survive electrical injury should be monitored in hospital if they have a history of cardio respiratory problems or have had:
- loss of consciousness
- cardiac arrest
- electro cardiographic abnormalities soft tissue damage and burns.
Severe burns (thermal or electrical), myocardialnecros is, the extent of central nervous system injury, and secondary multi system organ failure determine the morbidity and long-term prognosis. Bone marrow embolism has also been reported in some cases.633 There is no specific therapy for electrical injury, and the management is supportive. Prevention remains the best way to minimise the prevalence and severity of electrical injury.
Mass casualty incidents
Introduction
Mass casualty incidents(MCIs), characterised by greater demand for medical care than available resources, are rare events. Among the 19.8 million yearly emergency medical services (EMS) activations in the United States, 0.3% had an MCI code, but incidence of real disasters is much lower.634 The International Federation of Red Cross and Red Crescent Societies (IFRC) reports about 90 disasters in Europe and 650 events worldwide annually.635 The MCI or disaster can be caused by variety of chemical, biological, radiological or nuclear (CBRN) incidents, but traumatic incidents (e.g. traffic accidents, acts of crime, or natural and industrial disasters) play a leading role in developed countries.636 Initial triage of casualties enables identification of patient care priorities. Unlike normal circumstances, CPR is not usually initiated in MCI, in order to avoid delaying potentially effective treatment for salvageable victims. This critical decision depends on available resources in relation to the number of casualties.
Triage and decision-making on scene Safety.
- Safety at scene is paramount. Those first on scene must identify the actual and potential hazards and appropriate assistance must be requested immediately. The presence of multiple unconscious victims should always alert rescuers to the possibility of a CBRN Unexpected danger may be present at crime scenes, or places polluted by noxious substances e.g. carbon monoxide, industrial cyanides or other chemicals. Duringsar in attacks in Japan, 10% of 1363 EMS technicians developed poisoning, mostly from primary victims in poorly ventilated ambulances.637
- Use adequate protection measures and consider potential risks before approaching casualties. Be aware that wearing some personnel protective equipment may adversely affect performance of treatment interventions and limit the care that can Simulation studies have shown reduced success rate of advanced airway techniques, prolonged time for securing IV and IO access, and difficulties with drug preparation.638–640
Triage.
- Use a triage system to prioritise treatment, e.g. START (Simple Triage and Rapid Transport), Newport Beach Fire Department, CA, USA,641 SALT (Sort- Assess-Lifesaving Interventions–Treat/Transport).642, 643 Advanced prehospital teams involved in the initial scene triage must avoid over triage. Repeated triage (re-triage) is needed on entering the hospital and responsible personnel at all stages of emergency care must be familiar with the triage system used.
- If the START triage sieve is used, everyone able to walk is directed to clear the scene, and respiratory status of non-walking patients If the casualty does not breathe, open the airway using basic manoeuvers (head tilt and chin lift, or jaw thrust). Look, listen and feel for no more than 10s. A patient who does not begin breathing is triaged as dead. If an unresponsive victimis breathing normally, turn them into the recovery position and label as immediate (highest priority) for treatment. Further assessment of casualties, e.g. respiratory rate, capillary refill time, etc.,and depends on individual triage protocols.
- The decision to use an MCI triage sieve, and withhold CPR to those withimminent death (including victims without signs of life), is the responsibility of a medical commander who is usually the most experienced EMS clinician on scene.
- Triage inaccuracy may have fatal consequences in patients with survivable Healthcare professionals must be regularly trained to use the triage protocols during simulations and live exercises.644 Modern technologies such as educational video games enhance learning and improve subsequent performance when compared to traditional educational methods, e.g. cardsort exercise.645 Training allows fast and correct recognition of those requesting life-saving procedures, and reduces the risk of inappropriate care given to futile cases.
- Consider assigning a higher triage risk level to the elderly and to survivors of high- energy trauma in order to reduce prevent able After an aeroplanecrash in the Netherlands, 9% of the minor injuries (lowest priority), and 17% of all walking casualties were undertriaged while suffering serious injuries.646 In the National Trauma Database (NTDB), patients in all triage levels were compared to mortality outcomes. There were 322,162 subjects assigned to the lowest priority triage level of which 2046 died before hospital discharge. Age was the primary predictor of undertriage.641
- Perform life-saving interventions in patients triaged as immediate (highest priority) to prevent cardiac arrest: control major haemorrhage, open airway using basic techniques, perform chest decompression for tension pneumothorax, use antidotes, and consider initial rescue breaths in a non-breathing child.642
- In children, use of a special triage tapeora paediatric-specific MCI triage system (e.g. JumpSTART, Team Life Support, Inc., FL, USA, http://www.jumpstarttriage.com) or a universal SALT system.647 If it is not available, use any triage system for adults
C –SPECIAL PATIENTS
Cardiac arrest associated with concomitant diseases
Asthma
Introduction. Worldwide, approximately 300 million people of all ages and ethnic backgrounds have asthma.648 The worldwide prevalence of asthma symptoms ranges from 1 to 18% of the population with a high prevalence in some European countries (United Kingdom, Scandinavia and Netherlands) and in Australia.648,649 In recent years, the prevalence of asthma and its related morbidity and mortality appears to have plateaued and may even have decreased in some countries, especially in children and adolescents.650–653
The World Health Organization (WHO) has estimated that 15 million disability-adjusted life years(DALYs) are lost annually from asthma, representing 1% of the global disease burden. Annual worldwide deaths from asthma have been estimated at 250,000. The death rate does not appear to be correlated with asthma prevalence.648 National and international guidance for the management of severe asthma already exists.654 This guidance focuses on the treatment of patients with near-fatal asthma and subsequent cardiac arrest.
Patients at risk of asthma-related cardiac arrest. The risk of near. fatal asthma attacks is not necessarily related to asthma severity.655
Patients most at risk include those with:
- a history of near-fatal asthma requiring intubation and mechanical ventilation;656
- hospitalisation or emergency care for asthma in the past year;657
- low or no use of inhaled corticosteroids;658
- increasing use and dependence of beta-2 agonists;659
- anxiety, depressive disorders and/or poor compliance with therapy;660,661
- food allergy in a patient with 662
A national confidential enquiry carried out in the UK in 2014 showed that the majority of asthma-related deaths occurred before admission to hospital.663 Compared with younger adults, older adults have higher rates of near-fatal asthma-related events and higher comorbidity-adjusted risk of mortality.664
Causes of cardiac arrest. Cardiac arrest in a person with asthma is often a terminal event after a period of hypoxaemia; occasionally, it may be sudden. Cardiac arrest in those with asthma has been linked to:
- severe bronchospasm and mucous plugging. leading to asphyxia (this condition causes the. vast majority of asthma-related deaths);
- cardiac arrhythmias caused by hypoxia, which is the commonest cause of asthma-related 665 Arrhythmias can also be caused by stimulant drugs (e.g. beta-adrenergic agonists, aminophylline) or electrolyte abnormalities;
- dynamichyper inflation, i.e.autopositive end-expiratory pressure (auto-PEEP), can occur in mechanically ventilated Auto-PEEP is caused by air trapping and ‘breath stacking’ (air entering the lungs and being unable to escape). Gradual build-up of pressure occurs and reduces venous return and blood pressure;
- tension pneumothorax (often bilateral).
Diagnosis. Wheezing is a common physical finding, but severity does not correlate with the degree of airway obstruction. The absence of wheezing may indicate critical airway obstruction, whereas increased wheezing may indicate a positive response to bronchodilator therapy. SpO2 may not reflect progressive alveolar. hypoventilation, particularly if oxygen is being given. SpO2 may initially decrease during therapy because beta-agonists cause both bronchodilation and vasodilation, initially increasing intra pulmonary shunting.
Other causes of wheezing include: pulmonary oedema, chronic obstructive pulmonary disease (COPD), pneumonia, anaphylaxis,101 pneumonia, foreign bodies, pulmonary embolism, and subglottic mass.666
The severity of an asthma attack is defined in Table 4.3.
Prevention of cardiac arrest. A patient with severe asthma requires immediate and aggressive medical management to prevent deterioration. Base the assess ment and treatment on a systematic ABCDE approach. Patients with SpO2 < 92% or with features of life-threatening asthma are at risk of hypercapnia and require arterial blood gas measurement. Experienced clinicians should treat these high-risk patients in a critical-care area. The specific drugs and the treatment sequence will vary according to local practice.
Oxygen. Use a concentration of inspired oxygen that will achieve an SpO 94– 98%.127 High-flow oxygen by mask is some-2 times necessary. Lack of pulse oximetry should not prevent the use of oxygen.
Nebulised beta-2 agonists. Inhaled beta-2agonists are first line drugs inpatients with an acute asthma attack and should be administered as early as possible. Intravenous beta-2 agonists should be reserved for those patients in who inhaled therapy cannot be used reliably. Salbutamol, 5mg nebulised, is the corner stone of Table 4.3
The severity of asthma (PEF, peak expiratory flow) therapy for acute asthma in most of the world. Repeated doses every 15–20 min are often needed. Severe asthma may necessitate continuous nebulised salbutamol. Nebuliser units that can be driven by high-flow oxygen (at least 6L min−1) should be available.
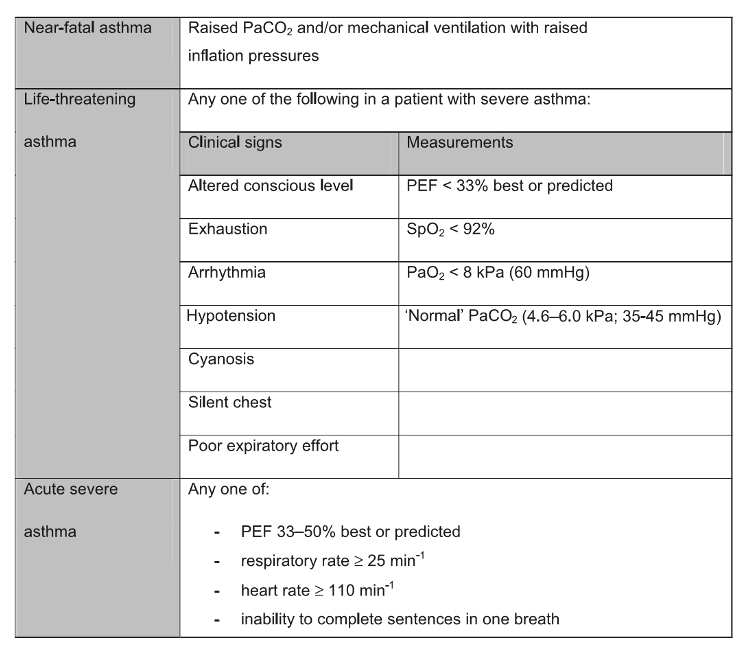
The hypoventilation associated with severe or near-fatal asthma may prevent effective delivery of nebulised drugs. If a nebuliser is not immediately available, beta-2 agonists can be temporarily administered by repeating activations of a metered dose inhaler via a large volume spacer device.667,668 Nebulised adrenaline does not provide additional benefit over and above nebulised beta-2 agonists in acute asthma.669
Nebulised anticholinergics. Nebulised anticholinergics (ipratropium, 0.5 mg 4–6 hourly) may produce additional bronchodilation in severe asthma or in those who do not respond to beta-agonists.670,671
Nebulised magnesium sulphate. Although limited evidence suggests that magnesium sulphate has some bronchodilator effects672 a review of 16 randomised or pseudo-randomised controlled trials in adults and children with acute asthma showed that inhaled magnesium alone or in combination with inhaled beta(2)-agonists (with or without inhaled ipratropium) was not associated with significant benefit in terms of improved pulmonary function or reduced hospital admissions.673 Results of small studies in adults with severe exacerbations of asthma showed improvements in pulmonary function with additional inhaled magnesium, however evidence wastoo limited to cometoa definite conclusion. Inhaled magnesium sulphate is currently not recommended for the treatment of acute asthma.
Intravenous magnesium sulphate. Studies of IV magnesium sulphate in acute severe and life-threatening asthma have produced conflicting results.672,674,675 A systematic review assessing 14 studies (three of which were multi centre trials) including a total of 2313 adult or mostly adult patients treated for acute asthma in the emergency department showed that a single infusion of 1.2 or 2g IV MgSO4 over 15–30min significantly reduced hospital admissions compared with placebo (odds ratio [OR] 0.75, 95% confidence interval[CI]0.60–0.92) and improved lung function.676 Participants in almost all of the studies had already been given at least oxygen, nebulised short- acting beta-2-agonists and IV corticosteroids in the emergency department. No difference was observed for other outcomes such as intensive care admissions and length of hospital stay.
Give a single dose of IV magnesium sulphate to patients with acute severe asthma (PEF < 50% best or predicted) who have not had a good initial response to inhaled bronchodilator therapy. The most commonly reported adverse effects of IV magnesium sulphate are flushing, fatigue, nausea, headache and hypotension.
Intravenous corticosteroids. Early use of systemic corticosteroids for acute asthma in the emergency department significantly reduces hospital admission rates, especially for those patients not receiving concomitant corticosteroid therapy.677 Although there is no difference in clinical effects between oral and IV formulations of corticosteroids,678 the IV route is preferable because patients with near-fatal asthma may vomit or beunable to swallow.
Intravenous bronchodilators. There is a lack of definitive evidence for or against the use of IV bronchodilators in this setting. Trials have primarily included spontaneously breathing patients with moderate-to life-threatening exacerbations of asthma; evidence in ventilated patients with life-threatening asthma or cardiac arrest is sparse. The use of IV bronchodilators should generally be restricted to patients unresponsive to nebulised therapy or where nebulised/inhaled therapy is not possible(e.g. a patient receiving bag-mask ventilation). A Cochrane review of intravenous beta-2 agonists compared with nebulised beta-2 agonists found no evidence of benefit and some evidence of increased side effects compared with inhaled treatment.679 Salbutamol may be given as either a slow IV injection (250 mcg IV slowly) or continuous infusion of 3– 20 mcg min−1.
Aminophylline. A Cochrane review of intravenous aminophylline found no evidence of benefit and a higher incidence of adverse effects (tachycardia, vomiting) compared with standard care alone.680,681 Whether aminophylline has a place as an additional therapy after treatment with established medications such as inhaled beta- agonists and systemic corticosteroids remains uncertain. If, after obtaining senior advice, the decision is taken to administer IV aminophylline, give a loading dose of 5 mg kg−1 over 20–30min (unless on maintenance therapy), followed by an infusion of 500–
700 mcg kg−1 h−1. Maintain serum theophylline concentrations below 20mcg mL−1 to avoid
toxicity. Leukotriene receptor antagonists. There are few data on the use of intravenous leukotriene receptor antagonists.682 Limited evidence suggests improvement of lung function and a non-significant trend towards reduced hospital admission when the intravenous leukotriene receptor antagonist montelukast was used as a rescue therapy in adults with acute asthma.683,684 Further studies are required to confirm the usefulness of leukotriene receptor antagonists in this setting. Intravenous fluids and electrolytes. Severe or near-fatal asthma is associated with dehydration and hypovolaemia, and this will further compromise the circulation in patients with dynamic hyperinflation of the lungs. If there is evidence of hypovolaemia or dehydration, give IV crystal loids. Beta-2 agonists and steroids may induce hypokalaemia, which should be monitored and corrected with electrolyte supplements as required.
Heliox. Heliox is a mixture of helium and oxygen (usually 80:20 or 70:30). A meta-analysis of four clinical trials did not support the use of heliox in the initial treatmentofpatientswithacute asthma.685
Intramuscular adrenaline. Sometimes it may be difficult to distinguish severe life-threatening asthma from anaphylaxis. Treat patients presenting with severe ‘asthma-like’ symptoms, but without pre-existing pulmonary disease (asthma, COPD), as if the cause was anaphylaxis. In these circumstances, administration of adrenaline 0.5 mg IM according to the anaphylaxis guidelines may be appropriate (see anaphylaxis).
Referral to intensive care. An intensive care specialist should assess patients that fail to respond to initial treatment, or develop signs of life-threatening asthma. Intensive care admission after asthma-related cardiac arrest is associated with significantly poorer outcomes compared with those in who a cardiac arrest does not occur.686
Consider rapid sequence induction and tracheal intubation if, despite efforts to optimise drug therapy, the patienthas:
- a decreasing conscious level, or coma;
- persisting or worsening hypoxaemia;
- deteriorating respiratory acidosis, despite intensive therapy;
- severe agitation, confusion and fighting against the oxygen mask
(clinical signs of hypoxaemia);
- progressive exhaustion; respiratory or cardiac arrest.
Elevation of the PCO2 alone does not indicate the need for tracheal intubation.687 Treat the patient, not the numbers. All patients transferred to intensive care units should be accompanied by a doctor suitably equipped and skilled to intubate.
Non-invasive ventilation. Non-invasive ventilation (NIV) decreases the intubation rate and mortality in COPD688; however, its role in patients with severe acute asthma is uncertain. There is insufficient evidence to recommend its routine use in asthma.689
Treatment of cardiac arrest.
Basic life support. Give BLS according to standard guidelines. Ventilation will be difficult because of increased airway resistance; try to avoid gastric inflation.
Advanced life support. Modifications to standard ALS guidelines include considering the need for early tracheal intubation. The peak airway pressures recorded during ventilation of patients with severe asthma(mean 67.8±11.1 cm H2O in 12 patients) are significantly higher than the normal lower oesophagealsphincter pressure (approximately 20cm H2O).690 There is a significant risk of gastric inflation and hypoventilation of the lungs when attempting to ventilate a severe asthmatic without a tracheal tube. During cardiac arrest this risk is even higher, because the lower oesophageal sphincter pressure is substantially less than normal.691
Respiratory rates of 8–10 breaths per minute and a tidal volume required for a normal chest rise during CPR should minimise dynamic hyperinflation of the lungs (air trapping).Tidal volume depends on inspiratory time and inspiratory flow. Lung emptying depends on expiratory time and expiratory flow. In mechanically ventilated severe asthmatics, increasing the expiratory time (achieved by reducing there spiratory rate) provides only moderate gains in terms of reduced gas trapping when a minute volume of less than 10L min−1 is used.690
Some case reports have reported ROSC in patients with air trapping when the tracheal tube was disconnected.692–696 If dynamic hyper inflation of the lungs is suspected during CPR, compression of the chest while disconnecting tracheal tube may relieve air trapping. Although this procedure is supported by limited evidence, it is unlikely to be harmful in another wise desperate situation.
Dynamic hyperinflation increases transthoracic impedance,697 but modern impedance-compensated biphasic defibrillation waveforms are no less effective in patientswith a higher impedance. As with standard ALS defibrillation protocols, consider increasing defibrillation energy if the first shock is unsuccessful and amanual defibrillator is available.
There is no good evidence for the use of open-chest cardiac compressions in patients with asthma-associated cardiac arrest. Working through the four Hs and four Ts will identify potentially reversible causes of asthma-related cardiac arrest. Tension pneumothorax can be difficult to diagnose in cardiac arrest; it may be indicated by unilateral expansion of the chest wall, shifting of the trachea and subcutaneous emphysema. Pleural ultrasound in skilled hands is faster and more sensitive than chestX-ray for the detection of pneumothorax.698 If a pneumothorax is suspected, perform needle decompression using a large gauge cannula and being careful to avoid direct puncture of the lung. Any attempt at needle decompression should be followed by insertion of a chest tube. Always consider bilateral pneumothoracesin asthma-related cardiac arrest (see tension pneumothorax).
ECLS can ensure both organ perfusion and gas exchange in cases of otherwise refractory respiratory and circulatory failure. Cases of successful treatment of asthma-related cardiac arrest in adults using ECLS have been reported699,700; however, the role of ECLS in cardiac arrest caused by asthma has never been investigated in controlled studies. The use of ECLS requires appropriate skills and equipment that may not be available in all hospitals.
Patients with ventricular assist devices
Introduction. All clinicians caring for patients with ventricular assist devices (VADs) should have received full training in the procedures for equipment failure and the cardiac arrest situation. The management of patients with VADs is more complex, in that a cardiac arrest may be due to mechanical failure and in this situation there may be actions specific to the device that are required. The use of external chest compression in patients with ventricular assist devices has been reviewed.701 There are isolated case reports of successful external chest compression without damage to the VAD. External chest compression may be particularly useful to decompress a non-functional right ventricle in cardiac arrests and often the right ventricle may be the cause of the loss of output.
Diagnosis of cardiac arrest. Confirming cardiac arrest in these patients may be difficult. A patient within vasive monitoring should be considered to have arrested if the arterial line reads the same asthecentralvenous pressure(CVP)line. Inpatients without invasive monitoring, if the patient has no signs of life and is not breathing, then they should be considered to have suffered a cardiac arrest. Transthoracic/transoesophagealecho cardiography (TTE/TOE), capnography or Doppler flowreadings in a major artery may assist in the diagnosis of whether there is meaningful perfusion. These devices also display pumpflow and this should be used to assist in a diagnosis of whether there has been a genuine loss of blood flow, or whether there is just alowflow situation with reduced conscious level.
Management of cardiac arrest. Patients with an implantable left ventricular assist devices (LVAD) such as a HeartMate (Thoratec, Pleasanton, CA, USA) or HeartWare (HeartWare, Framingham, MA, USA) device should have the same algorithm followed as the algorithm for arrest after cardiac surgery (see cardiac arrest following cardiac surgery). Check the rhythm; perform defibrillation for shockable rhythms (VF/pVT), start pacing for asystole. In pulseless electrical activity(PEA),turn the pacing off and verify there is no underlying VF, which must be treated by defibrillation. External chest compressions should be performed if immediate resuscitative efforts fail. Importantly, the airway and breathing checks should always be performed.
It is possible for a patient to have a systoleor VF, butstillhave adequate cerebral blood flow due to adequate and continued pump flow. If the patient is conscious and responding then you will have more time in which to resolve this arrhythmia and external chest compressions will not be needed.
Resternotomy should be performed in an established cardiac arrest within 10 days of surgery and after this time, either resternotomy or extracorporeal membrane oxygenation(ECMO) is areas on able option.
Causes of cardiac arrest. Cardiac arrest associated with acute neurological disease is relatively uncommon and can occur with subarachnoidhaemorrhage, intracerebral haemorrhage, epileptic seizures, and ischaemic stroke.702 In addition brain injury associated with trauma can cause cardiac arrest.
Cardiac arrest associated with neurological disease can be due to:
- Loss of consciousness, causing airway obstruction, hypoxaemia and respiratory. Loss of consciousness is also associated with an increased risk of aspiration of gastric contents into the lungs.
- Respiratory and cardiac depression caused by compression of the brainstem.
- Arrhythmias and myocardial dysfunction associated with acute neurological injury and inparticular sub-arachnoid haemorrhage.
- Sudden unexpected death in epilepsy (SUDEP) effects about 1 in every 1000 people with epilepsy.703
Neurological symptoms. Patients can have prodromal signs suggesting a neurological cause before cardiac arrest such as headache, seizures, impaired consciousness, and focal signs,704 but these are often non-specific and can include syncope, shortness of breath and chest pain. Cardiac or respiratory arrest occurs in between 3 and 11% of patents with subarachnoid haemorrhage,705 and the initial rhythm is usually non-shockable.
Treatment. Preventive measures for cardiac or respiratory arrest should be aimed at treating the underlying cause. Once cardiac arrest occurs, follow standard BLS and ALS guidelines. If ROSC is achieved, address the underlying cause in addition to standardpost resuscitation care.
Patients with subarachnoid haemorrhage may have ECG changes that suggest an acute coronary syndrome.704,706 Certain features such as a young age, female gender, non-shockable initial rhythm and neurological antecedents (e.g. headache, seizures, neurological deficits) are commonbutnon-specific for neurological cause.707 Individuals with neurological prodromal symptoms who achieve ROSC may be considered for CT brain scan. Whether this is done before or after coronary angiography will depend on clinical judgement regarding the likelihood of a subarachnoid haemorrhage versus acute coronary syndrome.
Outcome. Survival depends on the underlying cause and traditional factors (e.g. witnessed, bystander CPR) associated with survival.702 Prognosis is poor in those with ROSC after a subarach-noid haemorrhage.704,706,708 Individuals who achieve ROSC after a primary neurological cause of cardiac arrest will often fulfil neurological criteria for death and should be considered as potential organ donors.709
Obesity
Introduction. Worldwide obesity has more than doubled since 1980. In 2014, more than 1.9billion (39%) adults were overweight, and of these over 600 million(13%) were obese.
The World Health Organization (WHO) uses body mass index (BMI; weight in kg divided by height in m2) to define obesity in adults as710–712:
- overweight (25.0–29.9 kg m−2);
- obese (30.0–34.9 kg m−2);
- very obese (≥0kgm−2).
Many clinical studies have linked BMI to outcomes for a wide variety of cardio vascular and non-cardiovascular conditions.713–715
Traditional cardiovascular risk factors (hypertension, diabetes, lipid profile, prevalent coronary heart disease, heart failure, and left ventricular hypertrophy) are common in obese patient. Obesity is associated with increased risk of sudden cardiac death.715 Leading causes of death are dilated cardiomyopathy and severe coronary atherosclerosis.716
Modifications to cardiopulmonary resuscitation. No changes to sequence of actions are recommended in resuscitation of obese patients, but delivery of effective CPR may be challenging. Physical and physiological factors related to obesity may adversely affect the delivery of CPR, including patient access and transportation, patient assessment, difficult IV access, airway management, quality of chest compressions, the efficacy of vasoactive drugs, and the efficacy of defibrillation because none of these measures are standardised to a patient’s BMI or weight.710 More rescuers than usual may be required to assist in moving the patient and rescuer fatigue, particularly in relation to the delivery of chest compressions, may necessitate more regular changes of the rescuer than normal.
Chest compressions. As with all cardiac arrests, chest compressions are most effective when performed with the patient lying on a firm surface, but it may be unsafe for the patient and rescuers to attempt to move the patient down onto the floor. However, it is not always necessary in obese patients because the heavier torso sinks into the mattress, leaving less potential for mattress displacement during chest compression.717
In order to maintain sufficient depth of chest compresisons (approximately5cm but no more than 6 cm), rescuer fatigue may necessitate the need to change rescuers more frequently than the standard 2 min interval. Use of mechanical resuscitation devices is limited by the slope of the anterior chest wall, thoracic dimensions (sternum height up to 303 mm, and maximal width of 449 mm for piston devices (LUCAS); chest circumference up to 130cm and maximal chest width of 380mm for devices with a load-distributing band), and patient weight (up to 136 kg) (AutoPulse).
Defibrillation. Optimal defibrillation energy levels in obese patients are unknown. Unlike monophasic defibrillators, modern biphasic defibrillators are impedance-compensated and adjust their output according to the patient’s impedance. Two small retrospective studies have demonstrated no apparent weight- based influence on defibrillation efficacy,718 with a biphasic waveform of 150 J achieving high shock success rates without need for energy escalation.719 Defibrillation protocols for obese patients should therefore follow those recommended for patients with a normal BMI. Consider higher shock energies for defibrillation if initial defibrillation attempts fail.
Ventilation. Higher inspiration pressure is needed for positive pressure ventilation due to increased intra abdominal pressure.720
Early tracheal intubation by an experienced provider removes the need for prolonged bag-valve-mask ventilation, and may reduce any risk of aspiration. In all patients with extreme obesity, difficult intubation must be anticipated, with a clear failed intubation drill if necessary.721 If intubation fails, use of a supraglottic airway device (SAD) with oesop haegeal drain age tube is a suitable option.
Logistical considerations. A patient’s BMI should be considered when organising prehospital resuscitation, especially with regard to technical support and number of ambulance crew members.722
Special response vehicles modified to carry extremely obese patients, equipped withextra-wide interiors, reinforced stretchers and specialised lifting gear, should be used if possible. Weight limits of both stretchers and hospital beds must be checked prior to use.723 Underestimation of the technical aspects of rescue operations may cause secondary transportation trauma, or even prohibit safe transfer of obese patient to the hospital.722
Outcome. The relation between obesity and outcome from cardiac arrest is unclear. One large registry study has shown that survival from cardiac arrests caused by shockable rhythms (VF/pVT) was highest in overweight patients but was significantly lower in those who were very obese.710 In contrast, survival to discharge of non-shockable rhythms was similar across all BMI groups. Evidence from clinical cohort studies has suggested that overweight and obese patients may actually have a more favourable short-term and long-term prognosis than leaner patients once they are successfully resuscitated from cardiac arrest.711,724
Cardiac arrestassociatedwithpregnancy Introduction
Mortality related to pregnancy is relatively rare in Europe (estimate 16 per 100,000 live births) although there is a large variation between countries.725 The fetus must always be considered when an adverse cardio vascular event occurs in a pregnant woman. Fetal survival usually depends on maternal survival and initial resuscitation efforts should focus on the pregnant mother. Resuscitation guidelines for pregnancy are based largely on case series, extrapolation from non-pregnantarrests, manikin studies and expert opinion based on the physiology of pregnancy and changes that occur in normal labour.726,727
Significant physiological changes occur during pregnancy, e.g. cardiac output, blood volume, minute ventilation and oxygen consumption all increase. Furthermore, the gravid uterus can cause significant compression of iliac and abdominal vessels when the mother is in the supine position, resulting in reduced cardiac output and hypotension.
Causes of cardiac arrest
In developed regions, haemorrhage, embolism (thromboembolic and amniotic fluid), hypertensive disorders of pregnancy, abortion and genital tract sepsis account for most deaths directly associated with pregnancy, and pre-existing medical conditions for those indirectly related to pregnancy.728 A review of over 2 million pregnancies in the UK showed that maternal deaths (death during pregnancy, childbirth, or within 42 days after delivery) were associated with cardiac disease, neurological conditions, psychiatric conditions, and malignancies.729 A quarter of pregnant women who died in the UK had sepsis, and 1 in 11 had influenza. Pregnant women can also sustain cardiac arrest from the same causes as women of the same age group.
Prevention of cardiac arrest in pregnancy
In an emergency, use a systematic ABCDE approach. Many cardiovascular problems associated with pregnancy are caused by a orto-caval compression.
Treat a pregnant patient as follows:
- Place the patient in the left lateral position or manually and gently displace the
- Give oxygen, guided by pulse oximetry to correct any hypoxaemia.
- Give afluid bolus if there is hypotension or evidence of hypovolaemia
- Immediate lyre-evaluate the need for any drugs being given.
- Seek expert help Obstetric and neonatal specialists should be involved early in the resuscitation.
- Identify and treat the underlying cause, e.g. rapid recognition and treatment of sepsis, including early intravenous antibiotics.
Modifications to basic lifesupport
From 20 weeks’ gestation, theuterus can compress both the inferior venacava (IVC)and aorta, impeding venous return and cardiac output. Uterine obstruction of venous return can cause pre arrest hypotension or shock and, in the critically ill patient, may precipitate cardiac arrest.730,731 During cardiac arrest, the compromise invenous return and cardiac output by the graviduterus limits the effectiveness of chest compressions.
Non-arrest studies show that left lateral tilt improves maternal blood pressure, cardiac output and stroke volume732–734 and improves fetal oxygenation and heart rate.735–737 Non cardiac arrest data show that the gravid uterus can be shifted away from the IVC in most cases, by placing the patient in 15◦ of left lateral decubitus position.738 The value of relieving aortic or IVC compression during CPR is, however, unknown. Unless the pregnant victim is on a tilting operating table, left lateral tilt is not easy to perform whilst maintaining high-quality chest compressions. A variety of methods to achieve a left lateral tilt have been described including placing the victim on the rescuers knees,739 pillows or blankets, or the Cardiff wedge740 although their efficacy in actual cardiac arrests is unknown. Even when a tilting table is used, the angle of tilt is often overestimated.741 In a manikin study, the ability to provide effective chest compressions decreased as the angle of left lateral tilt increased and at an angle of greater than 30◦ the manikin tended to roll.740
The key steps for BLS in a pregnant patient are:
- Call for expert help early (including anobstetrician and aneonatologist).
- Strats BLS according to standard guidelines
- Ensure high-quality chest compressions with minimal interruptions.
- The hand position for chest compressions may need to be slightly higher on the g. third trimester.726
- Manually displace the uterus to the left to reduce IVC compression
- Add left lateral tilt if this is feasible and ensure the chest remains supported on a firm surface (e.g.in the operating room)–the Aim for between 15 and 30◦. Even a small amount of tilt may be better than no tilt. The angle of tilt used needs to enable high-quality chest compressions and if needed, allow Caes are an delivery of the fetus.
- Start preparing for emergency Caesarean section (see below)– the fetus will need to be delivered if initial resuscitation efforts fail.Modifications to advanced lifesupport
Defibrillation. For cardiac arrest with a shockable rhythm (VF/pVT) attempt defibrillation as soon as possible. There is no change in transthoracic impedance during pregnancy, suggesting that standard shock energies for defibrillation attempts should be used in pregnant patients.742 There is no evidence that shocks from a direct current defibrillator have adverse effects on the fetal heart.
Airway management. During pregnancy, there is a greater potential for gastro- oesophageal sphincter insufficiency and risk of pulmonary aspiration of gastric contents.743,744 Although pregnant patients are at risk of aspiration, oxygenation and ventilation is the priority over aspiration prevention. Early tracheal intubation will however make ventilation of the lungs easier in the presence of increased intra-abdominal pressure.
A tracheal tube 0.5–1 mm internal diameter(ID) smaller than that used for a non-pregnant woman of similar size may be necessary because of maternal airway narrowing from oedema and swelling.745 One study documented that the upper airways in the third trimester of pregnancy are narrower compared with their post partum state and to non-pregnant controls.746 Tracheal intubation may be more difficult in the pregnant patient.747 Expert help, a failed intubation drill and the use of alternative airway devices may be needed.748
Intravascular access. Early intravenous or intraosseous access will enabled rug and fluid administration. Aiming for access above the diaphragm may address any theoretical concerns associated with delayed circulation caused by IVC compression if drugs are infused through sites below the IVC.
Reversible causes
Rescuers should attempt to identify common and reversible causes of cardiac arrest in pregnancy during resuscitation (see special causes). The 4 Hs and 4 Ts approach helps identify all the common causes of cardiac arrest in pregnancy. Pregnant patients are also at risk of all the other causes of cardiac arrest for their age group (e.g. anaphylaxis, drug overdose, trauma).
Consider the use of abdominal ultrasound by a skilled operator to detect possible causes during cardiac arrest; however, do not delay other treatments and minimise interruptions to chest compressions.
Specific causes of cardiac arrest in pregnancy include the following:
Haemorrhage. Life-threatening haemorrhage can occur both antenatally and postnatally.728 Postpartum haemorrhage is the commonest single cause of maternal death worldwide and is estimated to cause one maternal death every 7min.749 Associations include ectopic pregnancy, placental abruption, placent apraevia, placenta accreta, and uterine rupture.750 A massive haemorrhage protocol must be used in all units and should be updated inconjunction with the blood bank. Women at high risk of bleeding should be delivered in centres with facilities for blood transfusion, intensive care and other interventions, and plans should be made inadvance for their management. Treatment is based on an ABCDE approach.
The key step is to stop the bleeding. Consider the following751,752:
- Fluid resuscitation, including use of rapid transfusion system and cell 753
- Oxytocin and prostaglandin analogues to correct uterine atony.754
- Massaging the uterus755
- Correction of coagulopathy including use of tranexamic acid and/or recombinant activated factor VII.756–758
- Uterine balloon tamponade or packing 759,760
- Uterine compression sutures 761
- Angiography and endovascular embolisation763,764
- Aortic cross-clamping in catastrophic 765
Cardiovascular disease. Myocardial infarction and aneurysm or dissection of the aorta or its branches, and peripartum cardiomyopathy cause most deaths from acquired cardiac disease.766–768
Patients with known cardiac disease need to be managed in a specialist unit. Pregnant women may develop an acute coronary syndrome, typically in association withrisk factors such as obesity, older age, higher parity, smoking, diabetes, pre-existing hypertension and a family history of ischaemic heart disease.750,769 Pregnant patients can have atypical features such as epigastric pain and vomiting. Percutaneous coronary intervention (PCI) is the reperfusion strategy of choice for ST-elevation myocardial infarction in pregnancy. Thrombolysis should be considered if urgent PCIis unavailable. A review of 200 cases of thrombolysis for massive pulmonary embolism in pregnancy reported a maternal death rate of 1% and concluded that thrombolytic therapy is reasonably safe in pregnancy.770
Increasing numbers of women with congenital heart disease are becoming pregnant.771 Heart failure and arrhythmias are the commonest problems, especially in those with cyanotic heart disease. Pregnant women with known congenital heart disease should be managed in specialist centres.
Pre-eclampsia and eclampsia. Eclampsia is defined as the development of convulsions and/or unexplained coma during pregnancy or postpartum in patientswith signs and symptoms of pre-eclampsia.772,773 Magnesium sulphate is effective in preventing approximately half of the cases of eclampsia developing in labour or immediately postpartum in women with preeclampsia.774–777 Use magnesium sulphate infusion for the treatment of eclampsia.778–781
Pulmonary embolism.The estimated incidence of pulmonary embolism is 1–1.5per 10,000 pregnancies, with a case fatality of 3.5% (95% CI 1.1–8.0%).782 Risk factors include obesity, increased age, and immobility. Successful use of fibrinolytics for massive, life-threatening pulmonary embolism in pregnant women has been reported.770,783–786
Amniotic fluid embolism. Amniotic fluid embolism (AFE) usually presents around the time of delivery with sudden cardiovascular collapse, breathlessness, cyanosis, arrhythmias, hypotension and haemorrhage associated with disseminated intravascular coagulopathy.787 Patients may have warning signs preceding collapse including breathlessness, chest pain, feeling cold, lightheadedness, distress, panic, a feeling of pins and needles in the fingers, nausea, and vomiting. The UK Obstetric Surveillance System (UKOSS) identified 120 cases of AFE between 2005 and 2014 with a total and fatal incidence estimated as 1.7 and 0.3 per 100,000, respectively, and association with older maternal age, multiple pregnancy, placenta praevia and induction of labour, instrumental vaginal and Caesarean delivery.788
Treatment is supportive, as there is no specific therapy based on an ABCDE approach and correction of coagulopathy. Successful use of extracorporeal life support techniques for women suffering life threatening amniotic fluid embolism during labour and delivery is reported.789
Peri-mortem delivery of the fetus
Consider the need for an emergency hysterotomy or Caesarean section as soon as a pregnant woman goes into cardiac arrest. In some circumstances immediate resuscitation attempts will restore a perfusing rhythm; in early pregnancy this may enable the pregnancy to proceed to term. Three observational studies of 154 subjects collectively790–792 provide very low quality evidence regarding the use of peri-mortem Caesarean section. Based on expert opinion, when initial resuscitation attempts fail, delivery of the fetus may improve the chances of successful resuscitation of the mother and fetus.793–795
One systematic review documented 38 cases of Caesareansectionduring CPR, with 34 surviving infants and 13 maternal survivors at discharge, suggesting that Caesarean section may have improved maternal and neonatal outcomes.796 The best survival rate for infants over 24–25 weeks’ gestation occurs when delivery of the infant is achieved within 5 min after the mother’s cardiac arrest.793,797–799 This requires that the provider commence the hysterotomy at about 4 min after cardiac arrest. At older gestational ages(30–38 weeks), infant survival is possible even when delivery was after 5 min from the onset of maternal cardiac arrest796 A case series suggests increased use of Caesarean section during CPR with team training791; in this series no deliveries were achieved within 5 min after starting resuscitation. Eight of the twelve women had ROSC after delivery, with two maternal and five newborn survivors. Maternalcasefatalityratewas83%. Neonatal case fatality rate was 58%.791
Delivery will relieve IVC compression and may improve chances of maternal resuscitation. The Caesarean delivery also enables access to the infant so that new born resus citation can begin.
Decision-making for emergency hysterotomy (Caesarean section). The gravid uterus reaches a size that will begin to compromise aorto-caval blood flow at approximately 20 weeks gestation; however, fetal viability begins at approximately 24–25 weeks.800
Portable ultrasound is available in some emergency departments and may aid in determination of gestational age (inexperienced hands) and positioning, provided its use does not delay the decision to perform emergency hysterotomy.801
- At gestational age less than 20 weeks, urgent Caesarean delivery need not be considered, because a gravid uterus of this size is unlikely to significantly
- At gestational age approximately 20–23 weeks, initiate emergency hysterotomy to enable successful resuscitation of the mother, not survival of the delivered infant, which is unlikely at this gestational age.
- At gestational age approximately ≥24–25 weeks, initiate emergency hysterotomy to save the life of both the mother and the infant.
Post resuscitation care Post resuscitation care should follow standard guidelines. Targeted temperature management (TTM) has been used safely and effectively in early pregnancy with fetal heart monitoring and resulted in favourable maternal and fetal outcome after a term delivery.802 Implantable cardioverter defibrillators (ICDs) have been used in patients during pregnancy.803
Preparation for cardiac arrest in pregnancy
ALS in pregnancy requires coordination of maternal resuscitation, Caesarean delivery of the fetus and new born resuscitation ideally within 5 min.
To achieve this, units likely to deal with cardiac arrest in pregnancy should:
- have plans and equipment in place for resuscitation of both the pregnant woman and new born
- ensure early involvement of obstetric, anaesthetic and neonatal teams
- ensure regular training in obstetric 804,805
Elderly people Epidemiology
More than 50% of people resuscitated from OHCAs in the United States are aged 65 years or older.806 The incidence of cardiac arrests in elderly people is likely to increase as the world population ages. The incidence of cardiac arrest increases withage. In males, the incidence of OHCA at 80 years of age is about seven times greater than at 40 years of age.807 In females above 70 years of age it is more than 40 times greater than in women below 45 years of age. Inan observational study on in-hospital cardiac arrest patients above 65 years of age accounted for 46% of the total hospital admissions in the study period and for 65% of the ward cardiac arrests.808 In this study, the incidence of arrests was more than twice that in the younger patient population (2.2 versus 1.0 per 1000 patient admissions).
Causes of cardiac arrest
The incidence of both coronary heartdisease andc hronic heart failure increases with age. As a consequence, elderly people have an increased incidence of cardiac causes of arrest.809 However, the proportion of deaths that are sudden (i.e. due to a primary ventricular arrhythmia) decreaseswithage, due to a parallel increase in the proportion of deaths due to other cardiovascular causes.810
The incidence of PEA as the first recorded rhythm increases significantly with age809,811; with a parallel decrease of the incidence of shockable rhythms (VF/pVT).812
Prevention
Deterioration of vital signs leading to cardiac arrest is detected less accurately in elderly patients, compared with younger patients.813 Clinical signs of acute life- threatening conditions such as sepsis,814 acute myocardial infarction815 or heart failure816 are often blunted or non-specific in elderly patients, resulting in less physiological aberration and a lower Modified Early Warning Score (MEWS) in the 4h preceding cardiac arrest.808
Treatment
Management of periarrest conditions. Ageing is associated with several pathophysiological changes that should be taken into account when managing peri-arrest conditions. Increasing age is associated with autonomic and baroreflex dysfunction and with myocardial stiffening which impairs early diastolic filling.817 In addition, elderly critically ill patients are often hypovolaemic due to a reduction of both fluid intake and urine-concentrating ability.818 These changes compromise the cardiovascular response to fluid loss or postural changes and increase the hypotensive effect of sedatives and other vasoactive drugs.Elderly patients are at increased risk of severe hypotension during emergency airway management.819
Atrial fibrillation is the most common supraventricular arrhythmia in the elderly. It often causes cardiovascular compromise due to loss of the atrial contribution for diastolic filling, particularly in the elderly who have reduced ventricular compliance. Hypotension and an increased heart rate may reduce coronary perfusion and precipitate cardiac ischaemia, which is more likely in an elderly populationwithagreaterincidenceofcoronaryarterydisease.
Older patients are more likely to develop apnea or respiratory depression following the administration of opioid or benzodiazepines.818 Their lower baseline oxygen tension also increases the risk of developing hypoxia.
Advancing age is associated with an increased rate of comorbidities. Elderly patients often take several medications, which may interfere with drugs administered inperi- arrest conditions. The incidence of adverse drug reactions in the elderly is 2–3 times higher than in younger patients.820
Management of cardiac. arrest. No modifications of standard resuscitation protocols are needed when managing aged patients in cardiac arrest. Rescuers, however, should be aware that the risk of both sternal and rib fractures is higher in elderly.821–823 The incidence of CPR-related injuries increases with duration of CPR.823
Outcome
Older age is associated with an increasingly lower short-term survival rate after cardiac arrest.824–829 In a large registry of OHCAs, survival to discharge was 8% for those aged 65–79 years, 4% for octogenarians and 2% for nonagenarians.826 In another study, the adjusted risk for 30-day mortality in elderly resuscitated comatose patients was 1.04 (95% CI 1.03–1.06) per year of age.812
Increasing age is also associated with lower long-term survival after resuscitation. In a retrospective cohort study on elderly patients discharged alive after CPR from in-hospital cardiac arrest the risk-adjusted rate of 1-year survival was63.7%, 58.6%, and 49.7% among patients 65–74, 75–84, and≥85 years of age, respectively (P <0.001).827 In another study patients ≥65 years of age discharged alive after resuscitation from VF/pVT cardiac arrest showed a significantly lower long-term survival than an age- and gender-matched controls, while this was not observed in younger resuscitated patients.830
In those who do survive, neurological outcome is good in elderly survivors of cardiac arrest, with 95% having a cerebral performance category (CPC) score of 1– 2 on discharge from ICU824 and 72% at hospital discharge.827
Decision to resuscitate
| PeterPaal | Speakers honorarium. Vida. care, Zoll |
| Ruud Koster | Medical advisor Physio Control and |
Elderly patients with cardiac arrest are significantly less likely to receive resuscitation than younger patients.831, 832 When deciding to resuscitate elderly patients, age alone should not be the only criterion to consider and other more established criteria, i.e. witnessed status, resuscitation times, and first recorded rhythm, are important factors.833 In addition, we suggest that pre-arrest factors, such as the degree of autonomy, quality of life, mental status and the presence of major comorbidities, should also be considered. Whenever possible, a decision to resuscitate or not, should be discussed in advance with the patient and his/her family (see ethics of resuscitation and end-of-life decisions).243
Collaborators
Alessandro Barelli, Intensive Care Medicine and Clinical Toxicology, Catholic University School of Medicine, Rome, Italy
Bernd W.Böttiger, Department of Anaesthesiology and Intensive Care Medicine, University Hospital. of Cologne, Cologne,Germany
Marios Georgiou, American Medical Center, Nicosia, Cyprus
Anthony J. Handley, Honorary Consultant Physician,Colchester,UK
Thomas Lindner, Department of Anaesthesiology and Intensive Care, Stavanger University Hospital, Stavanger, Norway; Norwegian Air Ambulance Foundation, Drøbak, Norway
Mark J. Midwinter, NIHR Surgical Reconstruction and Microbiology Research Centre, University. of Birmingham,UK
Koenraad G. Monsieurs, Emergency Medicine, Faculty of Medicine and Health Sciences, University of Antwerp, Antwerp, Belgium; Faculty of Medicine and Health Sciences, University of Ghent,
Ghent, Belgium; Emergency Medicine, Ghent University, Ghent, Belgium
Wolfgang A. Wetsch, Department of Anaesthesiology and Intensive Care Medicine, University. Hospital of Cologne, Cologne, Germany
Conflicts of interest
Anatolij Truhlárˇ No conflict of interest reported
Annette Alfonzo No conflict of interest reported
Carsten Lott No. conflict of interest reported
Charles D.Deakin Director Prometheus Medical Ltd
Claudio Sandroni No conflict of interest reported
David A. Zideman No conflict of interest reported
David J.Lockey No conflict of interest reported
Gamal Eldin Abbas Khalifa No conflict of interest reported
Gavin D.Perkins EditorResuscitation
Guttorm Brattebø ChairBESTfoundation
Hermann Brugger Medical advisor EURAC/ICAR alpine medicine
Jasmeet Soar Editor Resuscitation
Jerry P.Nolan Editor-in-Chief Resuscitation
Joel Dunning Speakers honorarium CARDICA
Joost J. L. M. Bierens Board member/Advisor KNRM; KNRD;
Life Saving societies
Karl-Christian Thies Chair EuropeanTraumaCourse Organisation ETCO
References
-
- Soar J, Perkins GD, Abbas G, et al. European Resuscitation Council Guidecilon Potassiumin Clinical Practice. Arch Intern Med 2000;160:2429–36.
- Safar P, Paradis NA, Weil MH. Asphyxial cardiac arrest. In: Paradis NA, Halperin 2014;15:58–63. HR, Kern KB, Wenzel V, Chamberlain DA, editors. Cardiac arrest – the science
- Farmery AD, Roe PG. A model to describe the rate of oxy haemoglobindesatu
- DeBehnke DJ, Hilander SJ, Doble rDW, Wickman LL, Swart GL. The hemody- 2014;15:104–11. namic and arterial blood gas response to. as phyxiation: a canine model of
- Deasy C, Bray J, Smith K, Bernard. S, Cameron P, Committee VS. Hanging-
- SOS-KANTO StudyGroup. Cardio pulmonary resuscitation by bystanders with chest compression only(SOS-KANTO): an observational study. Lancet 2007;369:920–6.
- Ogawa T, Akahane M, Koike. S, Tanabe S, Mizoguchi T, Imamura T. Out- Anaesth 998;45:317–23. comes of chest compression only. CPR versus conventional. CPR conducted.
- Deasy C, Bray J, Smith K, Harriss LR, Bernard SA, Cameron P. Paediatric hanging.
- Wee JH, Park KN, Oh SH, Youn CS, Kim HJ, Choi SP.Outcome analys is of cardiac
- Davies D, Lang M, Watts R. Paediatric hanging and strangulation injuries: a10-
- Penney DJ, Stewart AH, Parr MJ. Prognostic. outcome indicators following hang
- Wee JH, Park JH, Choi SP, Park KN. Outcomes of patients admitted for hanging
- Mahoney B, Smith W, Lo D, Tsoi K, Tonelli M, Clase C. Emergency interventions for hyperkalaemia. Cochrane Database SystRev 2005: CD 003235.
- Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its diopulmonary resuscitation of a hypothermic polytraumatised cardiac arrest significance in chronic kidney disease. Arch Intern Med 2009;169:1156–62. patient. Resuscitation 2012; 83:e1-2.
- Acker CG, Johnson JP, Palevsky PM, Greenberg A.Hyperkalemia in hospital
- Moranne O, Froissart M, Rossert J,etal. Timing of onset of CKD-related randomized, cross-over manikin study. Am J Emerg Med 2013; 31:384–9. metabolic complications. J Am Soc Nephrol 2009;20:164
- Lin CH, Tu YF, Chiang WC,Wu SY, Chang YH,Chi CH. Electrolyte abnormalities situation. Der Anaesthesist 2006; 55:314–24.
- Khanagavi J, Gupta T, Aronow WS, etal. Hyperkalemia among hospitalized model. Acad Emerg Med 2003;10:187–91. patients and association between duration of hyperkalemia and outcomes.
- Smellie WS. Spurious hyperkalaemia. BMJ 2007;334:693–5. coronary perfusion pressure?Anesth Analg 2000;90:69–73.
- Asirvatham JR, Moses V, Bjornson L. Errors in potassium measurement : alab-
- You JS, Park YS, Chung HS, etal. Evaluating the utility of rapid point-of- combined with active rewarming. Resuscitation 2001;50:301 care potassium testing for the early identification of hyperkalemia in patients
- UK Renal Association. Treatment of acute hyperkalaemia in adults. Clinical avalanche burial – a randomised prospective porcine pilot study. Resuscitation practice guidelines. London: UK Renal Association; 2014. 2013;84:239–43.
- Ahmed J, Weisberg LS. Hyperkalemia in dialysis patients. Semin Dial 2001;14:348–56.
- Surawicz B, Chlebus H, Mazzoleni A. Hemodynamic and electro cardiographic Care Med 2001; 29:1006–11. effects of hyperpotassemia. Differences in response to slow and rapid in creases
- An JN, Lee JP, JeonHJ, et al. Severe hyperkalemia requiring hospitalization: hypothermia: 2014 update.Wilderness Environ Med 2014; 25:S66–85. predictors of mortality. Crit Care 2012; 16: R225.
- Elliott MJ, Ronksley PE, Clase CM, Ahmed SB, Hemmelgarn BR. Management of hospital care: wet clothing removal or addition of avaporbarrier.Wilderness patients with acute hyperkalemia. Can Med Assoc J 2010; 182:1631–5. Environ Med 2015; 26:11–20.
- Apel J, Reutraku lS, Baldwin D. Hypoglycemia in the treatment of hyperkalemia within sulin in patients with end-stage renal disease. Clin Kidney J
- Alfonzo AV, Isles C, Geddes C, Deighan C.Potassium disorders–clinical spec Control, Philips, Zoll, CardiacScience, trum and emergency management. Resuscitation 2006; 70:10–25. Defibtech, Jolife. No conflict of interest reported.
- El-Sherif N, Turitto G. Electrolyte disorders and arrhythmogenesis. Cardiol J 2011; 18:233–45.
- Paice BJ, Paterson KR, Onyanga-Omara F, Donnelly T, Gray JM, Lawson DH. Record linkage study of hypokalaemia in hospitalized patients. Postgrad Med J 1986;62:187–91.
- Kjeldsen K. Hypokalemia and sudden cardiac death. Exp Clin Cardiol 2010;15:e96–9.
- Cohn JN, Kowey PR, Whelton PK, Prisant LM. New guidelines for potassium replacement in clinical practice: a contemporary review by the National Coun
- Brown DJ, Brugger H,Boyd J, Paal P. Accidental hypothermia. N Engl J Med electrolyte abnormalities, poisoning, drowning, accidental hypothermia, 2012; 367:1930–8.
- Pasquier M, Zurron N, Weith B,etal. Deepaccidental hypothermia with core trocution. Resuscitation 2010;81:1400–33. temperature below 24 degree scpresenting with vital signs. High Alt Med Biol
- Walpoth BH, Galdikas J, Leupi F, Muehlemann W, Schlaepfer P, Althaus U. and practice of resuscitation medicine. 2nd ed. Cambridge: Cambride Univer- Assessment of hypothermia with a new “tympanic” thermometer. J Clin Monit sity Ptress; 2007.p.969–93. 1994;10:91–6.
- Strapazzon G, Procter E, Paal P, Brugger H. Pre-hospital core temperature ration during apnoea. Br J Anaesth 1996;76:284–91. measurement in accidental and therapeutic hypothermia. High Alt Med Biol
- Brugger H, Oberhammer R, Adler-Kastner L, Beikircher W. Therate of cooling pulseles selectrical activity.Resuscitation 1995;30:169–75. during avalanche burial; a“Core”issue. Resuscitation 2009; 80:956–8.
- Lefrant JY, Muller L, deLaCoussaye JE, etal. Temperature measurement in associated out-of-hospital cardiac arrests in Melbourne, Australia. EmergMed intensive care patients: comparis on of urinary bladder, oesophageal, rectal, J2013;30:38–42.
- Robinson J, Charlton J, Seal R, Spady D, Joffres MR. Oesophageal, rectal, axillary, tympanic and pulmonary artery temperatures during cardiac surgery. Can J
- Wood S. Interactions between hypoxia and hypothermia. AnnuRevPhysiol by lay people in patients without of hospital cardiopulmonary arrest wit- 1991;53:71–85-
- Schneider SM, Hypothermia: from recognition to rewarming. Emerg Med Rep 2011; 342:c7106. 1992; 13:1–20.
- Gilbert M, Busund R, Skagseth A, Nilsen PA, Solbo JP. Resuscitation from associated out of hospital cardiac arrest in Melbourne, Australia: characteristics accidental hypothermia of 13.7 degrees C with circulatory arrest.Lancet and outcomes. Emerg Med J 2011;28:411–5. 2000; 355:375
- Lexow K. Severe accidental hypothermia: survival after 6 hours 30 minutes of arrest due to hanging injury. Am JEmerg Med 2012; 30:690–4. cardio pulmonary resuscitation. Arctic Med Res 1991;50:112–4.
- Boue Y, Lavolaine J, Bouzat P, Matraxia. S, Chavanon O, Payen JF. Neurologic year retrospective description of clinical factors and outcomes. Paediatr Child recovery from profound accidental hypothermia afte 15 hours of. cardio pul-Health 2011; 16:e78–81. monary resuscitation. Crit Care Med 2014; 42:e1 67–70-
- Gordon L, Paal P, Ellerton JA, Brugger H, Peek GJ, Zafren K. Delayed and intering injuries.Resuscitation 2002; 54:27–9. mittent CPR for severe accidental hypothermia.Resuscitation 2015;90:46
- Paal P, Milani M, BrownD, Boyd J, Ellerton J. Termination of cardio pulmonary injuries with decreased consciousness but without cardiac arrest. Am J Emerg resuscitation in mountain rescue. High Alt Med Biol 2012; 13: 200–8. Med 2013; 31:1666–70.
- Danzl DF, Pozos RS, Auerbach PS, et al. Multicenter hypothermia survey. Ann Emerg Med 1987;16:1042–55.
- Putzer G, Tiefenthaler W, Mair P, Paal P. Near-infrared spectroscopy during car-
- Nolan JP, Soar. J,Wenzel V, Paal. P. Cardiopulmonary resuscitation and manageized patients: causes, adequacy of treatment, and results of an attempt to ment of cardiac arrest. Nat Rev Cardiol 2012;9: 499–511. improve physician compliance with published therapy guidelines. Arch Intern
- Putzer G, Braun P, Zimmermann A, et al. LUCAS compared to manual cardiopulmonary resuscitation is more effective during helicopter rescue–a prospective,
- Paal P, Beikircher W, Brugger H. Avalanche emergencies. Review of the current 17. Lin CH, Tu. YF, Chiang WC,Wu SY, Chang YH, Chi CH. Electrolyte abnormalities situation. Der Anaesthesist 2006; 55: 314–24.
- Stoner J,Martin G, O’Mara K, Ehlers J, Tomlanovich M. Amiodarone and have kidney disease. Am J Emerg Med 2013; 31:487–93. bretyliumin the treatment of hypothermic ventricular fibrillation in acanine
- Krismer AC, Lindner KH, Kornberger R, et al. Cardiopulmonary resuscitation Arch Med Sci 2014; 10: 251–7. during severe hypothermia in pigs: does epinephrine or vasopressin increase
- Kornberger E, Lindner KH, Mayr VD, et al.Effects of epine phrine in a pigmodel or atory perspective for the clinician. NA m J Med Sci 2013; 5:255–9. of hypothermic cardiac arrest and closed-chest cardiopulmonary resuscitation
- Mattu A, Brady WJ, manifestations of with chronic kidney disease in the emergency department. Yonsei Med J hypothermia. Am JEmerg Med 2002; 20:3 14–26. 2014; 55:1348–53.
- Paal P, Strapazzon G, Braun P, et al. Factors affecting survival from
- Ujhelyi MR, Sims JJ, Dubin SA, Vender J, Miller AW. Defibrillation energy requirements and electrical heterogeneity during total body hypothermia. Crit
- Zafren K, Giesbrecht GG, Danzl DF, et al. Wilderness Medical Society practice in concentration of plasmaK. Am Heart J 1967;73:647–64. guidelines for the out-of-hospital evaluation and treatment of accidental
- Henriksson O, Lundgren PJ, Kuklane K, et al. Protection against cold inpre
- Brown D, Ellerton J, Paal P, Boyd J. Hypothermia evidence. Afterdrop,
- Lundgren P, Henriksson O, Naredi P, Bjornstig U. The effect of active warming
- Gruber E, Beikircher W, Pizzinini R, et al. Non-extra corporeal rewarming at
- Bouchama A, Knochel JP. Heatstroke. NEnglJ Med 2002; 346:1978–88.
- WapplerF.Malignanthyperthermia.EurJAnaesthesiol2001;18:632–52.
- WapplerF.Malignanthyperthermia.EurJAnaesthesiol2001;18:632
- EmpanaJP,SauvalP,DucimetiereP,TaffletM,CarliP,JouvenX.Increaseinout
- CorisEE,RamirezAM,VanDurmeDJ.Heatillnessinathletes:thedangerous humidityandexercise.SportsMed2004;34:9–16.
- GroganH,HopkinsPM.Heatstroke:implicationsforcriticalcareandanaes- 2002;88:700–7.
- BouchamaA,DeVolEB.Acid-basealterationsinheatstroke.IntensiveCareMed 2001;27:680–5.
- PeaseS,BouadmaL,KermarrecN,SchortgenF,RegnierB,WolffM.Earlyorgan timeandoutcomeinclassicheatstroke.IntensiveCareMed2009;35:1454–8.
- AkhtarM,JazayeriMR,SraJ,BlanckZ,DeshpandeS,DhalaA.Atrioventricularclinical,electrophysiological,andtherapeuticconsiderations.
- el-KassimiFA,Al-MashhadaniS,AbdullahAK,AkhtarJ.Adultrespiratorydis-tresssyndromeanddisseminatedintravascularcoagulationcomplicatingheat stroke.Chest1986;90:571–4.
- Waruiru C, Appleton R. Arch Dis Child2004;89:751–6.
- BergerJ,HartJ,MillisM,BakerAL.Fulminanthepaticfailurefromheatstrokerequiringlivertransplantation.JClinGastroenterol2000;30:429–31.
- Huerta-Alardin AL, Varon J, Marik PE. Bench-to-bedsidereview: rhabdomyol
- Hadad E, Weinbroum AA, Ben-Abraham R. Drug-induced hyperthermia and
- Halloran LL, Bernard DW. Management of drug-induced hyperthermia. Curr database review. J Allergy Clin Immunol 2010; 125, 1098–1104.e1. Opin Pediatr 2004; 16:211–5.
- Bouchama A, Dehbi M, Chaves-Carballo E. Cooling and hemodynamic manage-practical recommendations. Crit Care 2007; 11:R54.
- Armstrong LE, Crago AE, Adams R, Roberts WO, Maresh CM. Whole-bodycool-runners: comparison of two field therapies. Am J Emerg Med 1996;14:355–8.
- Nolan JP, Soar J, Cariou A, et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015 Section 5. Post-resuscitation care. Resuscitation 2015; 95:201–21.
- Horowitz BZ. The golden hour in heatstroke: use of iced peritoneal lavage. Am Med 1989;7:616–9. intravenous fluid in comatose survivors of out-of-hospital cardiac arrest: a preliminary report. Resuscitation 2003; 56:9–13.
- Bernard S, Buist M, Monteiro O, Smith K. Induced hypothermia using large 1996; 348:301–2. volume, ice-cold
- Al-Senani FM, Graffagnino C, Grotta JC, et al. A prospective, multicenter pilot Icy catheter following cardiac arrest. Resuscitation 2004; 62:143–50.
- Schmutzhard E, Engelhardt K, Beer R, et al. Safety and efficacy of a novel cooling device to control bodytemperature in neurologic patients: a prospective pilot study. Crit Care Med 2002; 30: 2481–8.
- Behringer W, Safar P, Wu X, et al. Veno-venous extra corporeal blood shunt mild hypothermia in dog experiments and review of cooling methods. Resuscitation 2002; 54:89–98.
- Hostler D, Northington WE, Callaway CW. High-dose diazepam facilitate scoreinfusion in healthy volunteers. Appl Physiol Nutr Meta b2009;34:582–6.
- Hadad E, Cohen-Sivan Y, Heled Y, Epstein Y. Clinical review: treatment of heat
stroke: should dantrolene be considered? Crit Care 2005; 9:86–91.
- Channa AB, Seraj MA, Saddique AA, Kadiwal GH, Shaikh MH, Samarkandi AH. Is dantrolene effective in heatstroke patients? Crit Care Med 1990; 18:290–2.
- Bouchama A, Cafege A, Devol EB, Labdi O, el-Assil K, Seraj M.Ineffective-dantrolene sodium in the treatment of heatstroke. Crit Care Med 1991; 19:176–80.
- Larach MG, Gronert GA, Allen GC, Brandom BW, Lehman EB. Clinical pre- treatment, and complications of malignant hyperthermia in North America from 1987 to 2006. Anesth Analg 2010; 110:498–507.
- Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F. Dantrolene–pharmacology, therapeutic use and new developments. Anaesthesia 2004; 59:364–73.
- Hall AP, Henry JA. Acute toxic effects of ‘Ecstasy’(MDMA) and related com-heikh A, Shehata YA, Brown SG, Simons FE. Adrenaline (epinephrine) for the treatment of anaphylaxis with and without shock. Cochrane Database Syst Rev intravascular 2008: CD 006312. intensive care ’Driscoll BR, Howard LS, Davison AG, et al. BTS guideline for emergency oxypounds: overview of pathophysiology and clinical management. BrJ Anaesth gen use in adult patients. Thorax 2008;63, vi1-68. 2006; 96:678–85.
- Eshel G, Safar P, Sassano J, Stezoski W. Hyperthermia-induced cardiac arrest in Resuscitation 1990; 20:129–43.
- Eshel G, Safar P, Radovsky A, Stezoski SW. Hyperthermia-induced cardiac and practical experience. Wilderness Environ Med 2015, http://dx.doi. arrest in monkeys: limited efficacy of standard CPR. Aviat Space Environ Med
- Zeiner A, Holzer M, Sterz F, et al. Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Arch Intern Med 2001;161:2007–12. in prehospital
- Muraro A, Roberts G, Worm M, et al. Anaphylaxis: guidelines from the European Academy of Allergy and Clinical Immunology. Allergy 2014;69:1026–45.
- Kleber C, Giesecke MT, Lindner T, Haas NP, Buschmann CT. Requirement for a rate of 6.8 degrees C per hour in a deeply hypothermic arrested patient. a structured algorithm in cardiac arrest following major trauma epidemi Resuscitation 2014; 85:e119–20.
- Brenner ML, Moore LJ, DuBose JJ, et al. A clinical series of resuscitative endovas-
- Simons FE, Ardusso LR, Bilo MB, et al. International consensus on (ICON) anaphylaxis. World Allergy Organ J 2014;7:9. but not
- ohansson SG, Bieber T, Dahl R, et al. Revised nomenclature for allergy for global use: report of the Nomenclature Review Committee of the World Allergy Organization, October 2003. J Allergy Clin Immunol 2004;113:832–6. combination of heat,
- oar J, Pumphrey R, Cant A, et al. Emergency treatment of anaphylactic reactions – guidelines for healthcare providers. Resuscitation 2008; 77:157–69. thesia. Br J Anaesth
- oar J. Emergency treatment of anaphylaxis in adults: concise guidance. Clin Med 2009;9: 181–5.
- anesar SS, Javad S, de Silva D, et al. The epidemiology of anaphylaxis in Europe: a systematic review. Allergy 2013;68:1353–61. dysfunction course, cooling
- uraro A, Roberts G, Clark A, et al. The management of anaphylaxis in childhood: position paper of the European academy of allergology and clinical immunology. Allergy 2007;62: 857–71. nodal reentry:
- arper NJ, Dixon T, Dugue P, et al. Suspected anaphylactic reactions associated
-
urner PJ, Gowland MH, Sharma V, et al. Increase in anaphylaxis-related hospitalizations but no increase in fatalities: an analysis of United Kingdom national anaphylaxis data, 1992–2012. J Allergy Clin Immunol 2015;135, 956–63.e1. seizures: an update.
-
orm M, Moneret-Vautrin A, Scherer K, et al. First European data from the network of severe allergic reactions (NORA). Allergy 2014;69:1397–404.
-
ibbison B, Sheikh A, McShane P, Haddow C, Soar J. Anaphylaxis admissions to UK critical care units between 2005 and 2009. Anaesthesia 2012; 67: 833–9.
-
umphrey RS. Fatal anaphylaxis in the UK, 1992–2001. Novartis Found Sympysis–an overview for clinicians. Crit Care 2005; 9:158–69. 2004;257:116–28, discussion 128–32, 157–60, 276–85.
-
onzalez-Perez A, Aponte Z, Vidaurre CF, Rodriguez LA. Anaphylaxis epi muscle rigidity: a practical approach. Eur J Emerg Med 2003; 10:149–54. demiology in patients with and patients without asthma: a United Kingdom
-
umphrey RS. Lessons for management of anaphylaxis from a study of fatal reactions. Clin Exp Allergy 2000;30:1144–50. ment in heatstroke:
-
ampson HA, Munoz-Furlong A, Campbell RL, et al. Second symposium on the definition and management of an aphylaxis: summary report– Second National ing of hyperthermic Institute of Allergy and Infectious Disease/Food Allergy and Anaphylaxis Net- work symposium. J Allergy Clin Immunol 2006;117:391–7.
-
hami S, Panesar SS, Roberts G, et al. Management of anaphylaxis: a systematic review. Allergy 2014;69:168–75.
-
umphrey RSH. Fatal posture in anaphylactic shock. J Allergy Clin Immunol 2003;112:451–2. JEmerg
-
isscher PK, Vetter RS, Camazine S. Removing bee stings. Lancet
-
impson CR, Sheikh A. Adrenaline is first line treatment for the emergency treatment of anaphylaxis. Resuscitation 2010;81:641–2.
-
empSF, Lockey RF, Simons FE. Epinephrine: the drug of choice for anaphylaxis. study to evaluate the feasibility and safety of using the Cool Gard System and A statement of the World Allergy Organization. Allergy 2008; 63:1061–70.
-
heikh A, Shehata YA, Brown SG, Simons FE. Adrenaline (epinephrine) for the treatment of anaphylaxis with and without shock. Cochrane Database Syst Rev intravascular 2008:CD006312. intensive care
-
autista E, Simons FE, Simons KJ, et al. Epinephrine fails to hasten hemody- namic recovery in fully developed canine anaphylactic shock. Int Arch Allergy Immunol 2002;128:151–64. cooling to induce
-
ong TT, Nelson MR, Chang JH, Engler RJ, Chowdhury BA. Adequacy of the epinephrine auto injector needle length in delivering epinephrine to the intra-muscular tissues. Ann Allergy Asthma Immunol 2005;94:539–42. cooling during cold saline
-
imons FE, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol 2001; 108:871–3.
-
imons FE, Roberts JR, Gu X, Simons KJ. Epinephrine absorption in children with a history of anaphylaxis. J Allergy Clin Immunol 1998; 101:33–7.
-
imons FE, Gu X, Johnston LM, Simons KJ. Can epinephrine inhalations be sub- stituted for epinephrine injection in children at risk for systemic anaphylaxis? Pediatrics 2000;106:1040–4. ness of
-
ompels LL, Bethune C, Johnston SL, Gompels MM. Proposed use of adrenaline (epinephrine) in anaphylaxis and related conditions: a study of senior house officers starting accident and emergency posts. Postgrad Med J 2002;78:416–8. sentation,
-
rown SG, Blackman KE, Stenlake V, Heddle RJ. Insect sting anaphylaxis; prospective evaluation of treatment with intravenous adrenaline and volume resuscitation. Emerg Med J 2004;21:149–54. a review of its
-
rown SG. Cardiovascular aspects of anaphylaxis: implications for treatment and diagnosis. Curr Opin Allergy Clin Immunol 2005;5:359–64.
-
’Driscoll BR, Howard LS, Davison AG, et al. BTS guideline for emergency oxy- pounds: overview of pathophysiology and clinical management. BrJ Anaesth gen use in adult patients. Thorax 2008;63, vi1-68. 2006;96:678–85.
-
heikh A, Ten Broek V, Brown SG, Simons FE. H1-antihistamines for the treat-
-
Choo KJ, Simons FE, Sheikh A. Glucocorticoids for the treatment of anaphylaxis. Cochrane Database Syst Rev 2010; 3: CD 007596.
-
Green R, Ball A. Alpha-agonists for the treatment of anaphylactic shock. Anaesthesia 2005; 60:621–2.
-
Kluger MT. The Bispectral Index during an anaphylactic circulatory arrest. Anaesth Intensive Care2001;29:544–7.
-
McBrien ME, Breslin DS, Atkinson S, Johnston JR. Use of methoxamine in the resuscitation of epinephrine- resistant electro mechanical dissociation. Anaesthesia 2001; 56:1085–9.
-
Rocq N, Favier JC, Plancade D, Steiner T, Mertes PM. Successful use of terlipressin in post-cardiac arrest resuscitation after an epinephrine-resistant anaphylactic shock to suxamethonium. Anesthesiology 2007; 107:166–7.
-
Kill C, Wranze E, Wulf H. Successful treatment of severe anaphylactic shock with vasopressin. Two case reports. Int Arch Allergy Immunol 2004; 134: 260–1.
-
Dewachter P, Raeth-Fries I, Jouan-Hureaux V, et al.A comparison of epinephrine only, arginine vasopressin only, and epinephrine followed by arginine vasopressin on the survival rate in a rat model of anaphylactic shock. Anesthesiology 2007; 106:977–83.
-
Higgins DJ, Gayatri P. Methoxamine in the management of severe anaphylaxis. Anaesthesia 1999; 54:1126.
-
Heytman M, Rainbird A. Use of alpha-agonists for management of anaphylaxis occurring under Heytman M, Rainbird A. Use of alpha-agonists for management of anaphylaxis occurring under anaesthesia: case studies and review. Anaesthesia 2004; 59:1210–5.
-
Schummer W, Schummer C, Wippermann J, Fuchs J. Anaphylactic shock: is vasopressin the drug of choice? Anesthesiology 2004; 101:1025–7.
-
DiChiara L, Stazi GV, Ricci Z, et al. Role of vasopressin in the treatment of anaphylactic shock in a child undergoing surgery for congenital heart disease: a case report. JMed Case Rep 2008; 2:36.
-
Meng L, Williams EL. Case report: treatment of rocuronium-induced anaphylactic shock with vasopressin. CanJ Anaesth 2008; 55:437–40.
-
Schummer C, Wirsing M, Schummer W. The pivotal role of vasopressin in refractory anaphylactic shock. Anesth Analg 2008;107:620–4.
-
Hiruta A, Mitsuhata H, Hiruta M, et al. Vasopressin may be useful in the treatment of systemic anaphylaxis in rabbits. Shock 2005; 24:264–9.
-
Thomas M, Crawford I. Best evidence topic report. Glucagon infusion in refractory anaphylactic shock in patients on beta-blockers. Emerg Med J 2005; 22:272–3.
-
Allen SJ, Gallagher A, Paxton LD. Anaphylaxis to rocuronium. Anaesthesia 2000; 55:1223–4.
-
Lafforgue E, Sleth JC, Pluskwa F, Saizy C. Successful extracorporeal resuscitation of a probable perioperative anaphylactic shock due to a tracurium. Ann Fr Anesth Reanim 2005; 24:551–5.
-
Vatsgar TT, Ingebrigtsen O, Fjose LO, Wikstrom B, Nilsen JE, Wik L. Cardiac arrest and resuscitation with an automatic mechanical chest compression device (LUCAS) due to anaphylaxis of a woman receiving caesarean section because of pre-eclampsia. Resuscitation 2006;68:155–9.
-
Schwartz LB. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am 2006;26:451–63.
-
Brown SG, Blackman KE, Heddle RJ. Can serum mast cell tryptase help diagnose anaphylaxis? Emerg Med Australas 2004;16:120–4.
-
Tole JW, Lieberman P. Biphasic anaphylaxis: review of incidence, clinical predictors, and observation recommendations. Immunol Allergy Clin North Am 2007;27:309–26,viii.
-
Simons FE, Lieberman PL, Read Jr EJ, Edwards ES. Hazards of unintentional injection of epinephrine from auto injectors: a systematic review. Ann Allergy Asthma Immunol 2009;102:282–7.
-
Campbell RL, Luke A, Weaver AL, et al. Prescriptions for self-injectable epinephrine and follow-up referral in emergency department patients presenting with anaphylaxis. Ann Allergy Asthma Immunol 2008; 101:631–6.
-
Kelso JM. A second dose of epinephrine for anaphylaxis: how often needed and how to carry. J Allergy Clin Immunol 2006; 117: 464–5.
-
Choo K, Sheikh A. Action plans for the long-term management of anaphylaxis: systematic review of effectiveness. Clin Exp Allergy 2007; 37:1090–4.
-
Zwingmann J, Mehlhorn AT, Hammer T, Bayer J, Sudkamp NP, Strohm PC. Survival and neurologic outcome after traumatic out-of-hospital cardiopulmonary arrest in a pediatric and adult population: a systematic review. Crit Care 2012;16:R117.
-
Leis CC, Hernandez CC, Blanco MJ, Paterna PC, Hernandez Rde E, Torres EC. Traumatic cardiac arrest: should advanced life support be initiated? J Trauma Acute Care Surg 2013;74:634–8.
-
Cureton EL, Yeung LY, Kwan RO, et al. The heart of the matter: utility of ultrasound of cardiac activity during traumatic arrest. JTrauma Acute Care Surg 2012; 73:102–10.
-
Engdahl J, Herlitz J. Localization of out-of-hospital cardiac arrest in Goteborg 1994–2002 and implications for public access defibrillation. Resuscitation 2005; 64:171–5.
-
Ong ME, Tan EH, Yan X, et al. An observational study describing the geographic time distribution of cardiac arrests in Singapore: what is the utility of geographic information systems for planning public access defibrillation (PADSPhaseI). Resuscitation 2008; 76:388–96.
-
Stratton SJ, Brickett K, Crammer T. Prehospital pulseless, unconscious penetrating trauma victims: field assessments associated with survival. JTrauma 1998; 45:96–100.
-
Cera SM, Mostafa G, Sing RF, Sarafin JL, Matthews BD, Heniford BT. Physiologic predictors of survival in post-traumatic arrest. Am Surg 2003; 69:140–4.
-
Powell DW, Moore EE, Cothren CC, etal. Is emergency department resuscitative thoracotomy futile care for the critically injured patient requiring prehospital cardio pulmonary resuscitation? J Am CollSurg 2004; 199:211–5.
-
Esposito TJ, Jurkovich GJ, Rice CL, Maier RV, Copass MK, Ashbaugh DG. Reappraisal of emergency room thoracotomy in a changing environment. JTrauma 1991;31:881–5, discussion 885–7.
-
Martin SK, Shatney CH, Sherck JP, et al. Blunt trauma patients with prehospital pulseless electrical activity(PEA): poor ending assured. JTrauma 2002;53:876–80, discussion 880–1.
-
Millin MG, Galvagno SM, Khandker SR, et al. Withholding and termination of resuscitation of adult cardiopulmonary arrest secondary to trauma: resource document to the joint NAEMSP-ACSCOT position statements. JTraumaAcute Care Surg 2013; 75:459–67.
-
Lockey DJ, Lyon RM, Davies GE. Development of a simple algorithm to guide the effective management of traumatic cardiac arrest. Resuscitation 2013;84:738–42.
-
Sherren PB, Reid C, Habig K, Burns BJ. Algorithm for the resuscitation of traumatic cardiac arrest patients in a physician-staffed helicopter emergency medical service. Crit Care 2013; 17:308.
- Smith JE, Rickard A, Wise D. Traumatic cardiac arrest. J R Soc Med 2015; 108:11–6.
- Soar J, Nolan JP, Bottiger BW, et al. European Resuscitation Council Guidelines for Resuscitation 2015 Section 3. Adult advanced lifesupport. Resuscitation 2015; 95:99–146.
- Luna GK, Pavlin EG, Kirkman T, Copass MK, Rice CL. Hemodynamic effects of external cardiac massage in trauma shock. JTrauma 1989; 29:1430–3.
- Willis CD, Cameron PA, Bernard SA, Fitzgerald M. Cardiopulmonary resuscitation after traumatic cardiac arrest is not always futile. Injury 2006; 37:448–54.
- Lockey D, Crewdson K, Davies G. Traumatic cardiac arrest: who are the survivors? Ann Emerg Med 2006; 48:240–4.
- Crewdson K, Lockey D, Davies G. Outcome from paediatric cardiac arrest associated with trauma. Resuscitation2007;75:29–34.
- Spahn DR, Bouillon B, Cerny V, et al. Management of bleeding and coagulopathy following major trauma: an updated European guideline. Crit Care 2013;17:R76.
- Kwan I, Bunn F, Chinnock P, Roberts I. Timing and volume of fluid administration for patients with bleeding. Cochrane Database Syst Rev2014;3: CD 450022.
- Bickell WH, Wall Jr MJ, Pepe PE, et al. Immediate versus delayed fluid resuscitation for hypotensive patients with penetrating torso injuries. NEnglJMed 1994; 331:1105–9.
- Harris T, Thomas GO, Brohi K. Early fluid resuscitation in severe trauma. BMJ 2012;345:e5752.
- Jansen JO, Thomas R, Loudon MA, Brooks A. Damage control resuscitation for patients with major trauma. BMJ2009;338:b1778.
- Holcomb JB, Tilley BC, Baraniuk S, et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs a 1:1:2 ratio and mortality in patients with severe trauma: the PROPPR randomized clinical trial. JAMA2015;313:471–82.
- Bodnar D, Rashford S, Hurn C, et al.Characteristics and outcomes of patients administered blood in the prehospital environment by a road based trauma response team. Emerg Med J 2013. May 5. [Epuba head of print].
- Lockey DJ, Weaver AE, Davies GE. Practical translation of hemorrhage control techniques to the civilian trauma scene. Transfusion 2013; 53:17S–22S.
- Holcomb JB, Jenkins D, Rhee P, et al. Damage control resuscitation: directly addressing the early coagulopathy of trauma. JTrauma 2007;62: 307–10.
- CRASH-2 collaborators Roberts I, Shakur H, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet 2011;377:1096–101,1101.e1–2.
- Cobas MA, De la Pena MA, Manning R, Candiotti K, Varon AJ. Prehospital intubations and mortality: a level 1 traumacenter perspective. Anesth Analg 2009;109:489–93.
- Lockey DJ, Healey B, Crewdson K, Chalk G, Weaver AE, Davies GE. Advanced airway management is necessary in prehospital trauma patients. BrJ Anaesth 2015;114:657–62.
- Pepe PE, Roppolo LP, Fowler RL. The detrimental effects of ventilation during low-blood-flow states. Curr Opin Crit Care 2005; 11:212–8.
- Escott ME, Gleisberg GR, Kimmel K, Karrer A, Cosper J, Monroe BJ. Simple thoracostomy. Moving beyong needle decompression intraumatic cardiac arrest. JEMS2014;39:26–32.
- Deakin CD, Davies G, Wilson A. Simple thoracostomy avoids chest drain in sertion in prehospital trauma. JTrauma 1995; 39:373–4.
- Flaris AN, Simms ER, Prat N, Reynard F, Caillot JL, Voiglio EJ. Clam shell incision versus left anterolateral thoracotomy. Which one is faster when performing a resuscitative thoracotomy The tortoise and the hare revisited. WorldJSurg 2015;39:1306–11.
- Wise D, Davies G, Coats T, Lockey D, Hyde J, Good A. Emergency thoracotomy: “how to do it”. Emerg Med J 2005; 22:22–4.
- Rhee PM, Acosta J, Bridgeman A, Wang D, Jordan M, Rich N. Survival after emergency department thoracotomy: review of published data from the past 25 years. J Am Coll Surg 2000;190:288–98.
- Burlew CC, Moore EE, Moore FA, et al. Western Trauma Association critical decisions in trauma: resuscitative thoracotomy. JTrauma Acute CareSurg 2012; 73:1359–63.
- Matsumoto H, Mashiko K, Hara Y, et al. Role of resuscitative emergency field thoracotomy in the Japanese helicopter emergency medical service system. Resuscitation 2009;80:1270–4.
- Seamon MJ, Chovanes J, Fox N, et al. The use of emergency department thoracotomy for traumatic cardiopulmonary arrest.Injury 2012;43:1355–61.
- Gao JM, Gao YH, Wei GB, et al. Penetrating cardiac wounds: principles for surgical management.World J Surg 2004;28:1025–9.
- Manz E, Nofz L, Norman A, Davies GE. Incidence of clotted heamopericardium in traumatic cardiac arrest in 152 thoracotomy patients. Scand J Trauma Resusc Emerg Med 2013;22:P20.
- Ferrada P, Wolfe L, Anand RJ, et al. Use of limited transthoracic echocardiography in patients with traumatic cardiac arrest decreases the rate of nontherapeutic thoracotomy and hospital costs. J Ultrasound Med 2014;33:1829–32.
- Walcher F, Kortum S, Kirschning T, Weihgold N, Marzi I. Optimized management of polytraumatized patients by prehospital ultrasound. Unfallchirurg 2002;105:986–94.
- Huber-Wagner S, Lefering R, Qvick LM, et al. Effect of whole-body CT during trauma resuscitation on survival: a retrospective, multicentre study. Lancet 2009;373:1455–61.
- Barton ED. Tension pneumothorax. Curr Opin Pulm Med 1999;5:269–74.
- Roberts DJ, Leigh-Smith S, Faris PD, et al. Clinical presentation of patients with tension pneumothorax: a systematic review. Ann Surg 2015. Jan 5.[Epubahead of print].
- Leigh-Smith S, Harris T.Tension pneumothorax–time for are-think? Emerg Med J 2005;22:8–16.
- Chen KY , Jerng JS, Liao WY , et al. Pneumothorax in the ICU: patient outcomes and prognostic factors. Chest 2002;122:678–83.
- Warner KJ, Copass MK, Bulger EM. Paramedic use of needle thoracostomy in the prehospital environment. Prehosp Emerg Care 2008;12:162–8.
- Mistry N, Bleetman A, Roberts KJ. Chest decompression during the resuscitation of patients in prehospital traumatic cardiac arrest. Emerg Med J 2009;26:738–40.
- Clemency BM, Tanski CT, Rosenberg M, May PR, Consiglio JD, Lindstrom HA. Sufficient catheter length for pneumothorax needle decompression: a metaanalysis. Prehosp Disaster Med 2015;30:249–53.
- Holcomb JB, McManus JG, Kerr ST, Pusateri AE. Needle versus tube thoracostomy in a swine model of traumatic tension hemopneumothorax. Prehosp Emerg Care 2009; 13:18–27.
- Massarutti D, Trillo G, Berlot G, et al. Simple thoracostomy in prehospital trauma management is safe and effective: a2-year experience by helicopter emergency medical crews. EurJ Emerg Med 2006;13:276–80.
- Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. EurHeartJ 2014;35:3033–69,69a–69k.
- Heradstveit BE, Sunde K, Sunde GA, Wentzel-Larsen T, Heltne JK. Factors complicating interpretation of capnography during advanced life support in cardiac arrest – a clinical retrospective study in 575 patients.Resuscitation 2012;83:813–8.
- Kurkciyan I, Meron G, Behringer W, et al. Accuracy and impact of presumed cause in patients with cardiac arrest. Circulation 1998;98:766–71.
- Kurkciyan I, Meron G, Sterz F, et al. Pulmonary embolism as a cause of cardiac arrest: presentation and outcome. Arch Intern Med 2000; 160: 1529–35.
- Pokorna M, Necas E, Skripsky R, Kratochvil J, Andrlik M, Franek O. How accurately can the aetiology of cardiac arrest be established in an out-of-hospital setting? Analysis by “concordance in diagnosis cross checktables”. Resuscitation 2011; 82:391–7.
- Wallmuller C, Meron G, Kurkciyan I, Schober A, Stratil P, Sterz F. Causes of in-hospital cardiac arrest and influence on outcome.Resuscitation 2012;83:1206–11.
- Bergum D, Nordseth T, Mjolstad OC, Skogvoll E, Haugen BO. Causes of in hospital cardiac arrest –incidences and rate of recognition. Resuscitation 2015; 87:63–8.
- Bottiger BW, Arntz HR, Chamberlain DA, et al. Thrombolys is during resuscitation for out-of- hospital cardiac arrest. N Engl J Med 2008; 359:2651–62.
- Silfvast T. Cause of death in unsuccessful prehospital resuscitation. J Intern Med 1991;229:331– 5.
- Heit JA, Silverstein MD, Mohr DN, Petterson TM, O’Fallon WM, Melton III LJ. Risk factors for deep vein thrombosis and pulmonary embolism: a population-based case–control study. Arch Intern Med 2000; 160:809–15.
- White RH. The epidemiology of venous thromboembolism. Circulation 2003;107:I4–8.
- Geibel A, Zehender M, Kasper W, Olschewski M, Klima C, Konstantinides SV. Prognostic value of the ECG on admission in patients with acute major pulmonary embolism. Eur Respir J 2005;25:843–8.
- Torbicki A, Pruszczyk P. The role of echo cardiography in suspected and established PE. Semin Vasc Med2001; 1:165–74.
- MacCarthy P, Worrall A, McCarthy G, Davies J. The use of transthoracic echocardiography to guide thrombolytic therapy during cardiac arrest due to massive pulmonary embolism. Emerg Med J 2002;19:178–9.
- Legome E, Pancu D. Future applications for emergency ultrasound. Emerg Med Clin North Am 2004;22:817–27.
- Roy PM, Colombet I, Durieux P , Chatellier G, Sors H, Meyer G. Systematic review and meta-analysis of strategies for the diagnosis of suspected pulmonary embolism.BMJ2005;331:259.
- Bova C, Greco F, Misuraca G, et al. Diagnostic utility of echocardiography in patients with suspected pulmonary embolism. Am J Emerg Med 2003;21:180–3.
- Li X, Fu QL, Jing XL, et al. A meta-analysis of cardiopulmonary resuscitation with and without the administration of thrombolytic agents. Resuscitation 2006;70:31–6.
- Janata K, Holzer M, Kurkciyan I, et al. Major bleeding complications in cardiopulmonary resuscitation: the place of thrombolytic therapy in cardiac arrest due to massive pulmonary embolism. Resuscitation 2003; 57:49–55.
- Böttiger BW, Martin E. Thrombolytic therapy during cardiopulmonary resuscitation and the role of coagulation activation after cardiac arrest. Curr Opin Crit Care2001;7:176–83.
- Fatovich DM, Dobb GJ, Clugston RA. A pilot randomised trial of thrombolys is in cardiac arrest (TheTICAtrial). Resuscitation 2004; 61:309–13.
- Konstantinov IE, Saxena P, Koniuszko MD, Alvarez J, Newman MA. Acute massive pulmonary embolism with cardiopulmonary resuscitation: management and results. Tex Heart Inst J 2007; 34:41–5, discussion 45–6.
- Zahorec R. Rescue systemic thrombolysis during cardiopulmonary resuscitation. Bratisl Lek Listy 2002;103:266–9.
- Lederer W, Lichtenberger C, Pechlaner C, Kroesen G, Baubin M. Recombinant tissue plasminogen activator during cardiopulmonary resuscitation in 108 patients with out-of-hospital cardiac arrest. Resuscitation 2001;50:71–6.
- Spöhr F, Böttiger BW. Safety of thrombolysis during cardiopulmonary resuscitation. Drug Saf 2003; 26:367–79.
- Wu JP, Gu DY, Wang S, Zhang ZJ, Zhou JC, Zhang RF. Good neurological recovery after rescue thrombolysis of presumed pulmonary embolism despite prior 100 minutes CPR. J Thorac Dis 2014;6:E289–93.
- Maj G, Melisurgo G, De Bonis M, Pappalardo F. ECLS management in pulmonary embolism with cardiac arrest: which strategy is better?Resuscitation 2014;85:e175–6.
- Swol J, Buchwald D, Strauch J, Schildhauer TA. Extracorporeal life support (ECLS) for cardiopulmonary resuscitation (CPR) with pulmonary embolism in surgical patients – a case series. Perfusion 2015. Apr 23. pii: 0267659115583682.[Epubaheadofprint].
- Doerge HC, Schoendube FA, Loeser H, Walter M, Messmer BJ. Pulmonary embolectomy: review of a 15-year experience and role in the age of thrombolytic therapy. Eur J Cardiothorac Surg 1996; 10:952–7.
- Fava M, Loyola S, Bertoni H, Dougnac A. Massive pulmonary embolism: percutaneous mechanical thrombectomy during cardiopulmonary resuscitation. J Vasc Interv Radiol 2005; 16:119–23.
- Hashiba K, Okuda J, Maejima N, et al. Percutaneous cardiopulmonary support in pulmonary embolism with cardiac arrest. Resuscitation2012;83:183–7.
- MillerAC,RosatiSF,SuffrediniAF,SchrumpDS.Asystematicreviewandpooled analysisof CPR-associatedcardiovascularandthoracicinjuries.Resuscitation 2014;85:724–31.
- Smekal D, Lindgren E, Sandler H, Johansson J, Rubertsson S. CPR-related injuries after manual or mechanical chest compressions with the LUCAS device: a multicentre study of victims after unsuccessful resuscitation. Resuscitation 2014;85:1708–12.
- Truhlar A, Hejna P, Zatopkova L, Skulec R, Cerny V. Concerns about safety of the Auto Pulse use in treatment of pulmonary embolism. Resuscitation 2012;83:e133–4, discussione 135.
- Nikolaou NI, Arntz HR, Bellou A, Beygui F, Bossaert LL, Cariou A. European Resuscitation Council Guidelines for Resuscitation 2015 Section 5. Initial management of acute coronary syndromes. Resuscitation 2015.
- Bossaert L, Perkins GD, Askitopoulou H, et al. European Resuscitation Council Guidelines for Resuscitation 2015 Section 11. The ethics of resuscitation and end-of-life decisions. Resuscitation2015;95:301–10.
- Lamhaut L, Jouffroy R, Soldan M, et al. Safety and feasibility of prehospital extra corporeal life support implementation by non-surgeons for out-of-hospital refractory cardiac arrest. Resuscitation2013;84:1525–9.
- Maekawa K, Tanno K, Hase M, Mori K, Asai Y. Extra corporeal cardio pulmonary resuscitation for patients without-of-hospital cardiac arrest of cardiac origin: a propensity-matched study and predictor analysis. Crit Care Med 2013;41:1186–96.
- Sakamoto T, Morimura N, Nagao K, et al. Extra corporeal cardiopulmonary resuscitation versus conventional cardiopulmonary resuscitation in adults with out-of-hospital cardiac arrest: a prospective observational study. Resuscitation 2014;85:762–8.
- Wagner H, Terkelsen CJ, Friberg H, et al. Cardiac arrest in the catheterisation laboratory:a5-year experience of using mechanical chest compressions to facilitate PCI during prolonged resuscitation efforts. Resuscitation 2010;81:383–7.
- Forti A, Zilio G, Zanatta P , et al. Full recovery after prolonged cardiac arrest and resuscitation with mechanical chest compression device during helicopter transportation and percutaneous coronary intervention. J Emerg Med 2014;47:632–4.
- Stub D, Bernard S, Pellegrino V , et al. Refractory cardiac arrest treated with mechanical CPR, hypothermia, ECMO and early reperfusion (the CHEER trial). Resuscitation2015;86:88–94.
- Belohlavek J, Kucera K, Jarkovsky J, et al. Hyperinvasive approach to out-of hospital cardiac arrest using mechanical chest compression device, prehospital intraarrest cooling, extracorporeal life support and early invasive assessment compared to standard of care. A randomized parallel groups comparative study proposal. “Prague OHCA study”. J Transl Med 2012;10:163.
- Stub D, Nehme Z, Bernard S, Lijovic M, Kaye DM, Smith K. Exploring which patients without return of spontaneous circulation following ventricular fibrillation out-of-hospital cardiac arrest should be transported to hospital?Resuscitation 2014;85:326–31.
- Mowry JB, Spyker DA, Cantilena Jr LR, McMillan N, Ford M. 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. ClinToxicol(Phila)2014;52: 1032–283.
- Zimmerman JL. Poisonings and overdoses in the intensive care unit: general and specific management issues. Crit Care Med 2003; 31:2794–801.
- Park JH, Shin SD, Song KJ, Park CB, Ro YS, Kwak YH. Epidemiology and outcomes of poisoning-induced out-of-hospital cardiac arrest. Resuscitation 2012; 83:51–7.
- Gunja N, Graudins A. Management of cardiac arrest following poisoning. Emerg Med Australas 2011;23:16–22.
- Yanagawa Y, Sakamoto T, Okada Y. Recovery from a psychotropic drug overdose tends to depend on the time from ingestion to arrival, the Glasgow Coma Scale, and a sign of circulatory insufficiency on arrival. Am J Emerg Med 2007;25:757–61.
- Thompson TM, Theobald J, Lu J, Erickson TB. The general approach to the poisoned patient. Dis Mon2014;60:509–24.
- Engebretsen KM, Kaczmarek KM, Morgan J, Holger JS. High-dose insulin therapy in beta- blocker and calcium channel-blocker poisoning. ClinToxicol(Phila) 2011;49:277–83.
- Cave G, Harvey MG. Should we consider the infusion of lipid emulsion in the resuscitation of poisoned patients? Crit Care 2014;18:457.
- Ozcan MS, Weinberg G. Intravenous lipidemulsion for the treatment of drug toxicity. J Intensive CareMed2014;29:59–70.
- Agarwala R, Ahmed SZ, Wiegand TJ. Prolonged use of intravenous lipid emulsion in a severe tricyclic anti depressant overdose. J Med Toxicol 2014;10:210–4.
- Kundu R, Almasri H, Moza A, Ghose A, Assaly R. Intravenous lipid emulsion in wide complex arrhythmia with alternating bundle branch block pattern from cocaine overdose. Kardiol Pol 2013;71:1073–5.
- de Lange DW, Sikma MA, Meulenbelt J. Extracorporeal membrane oxygenation in the treatment of poisoned patients. Clin Toxicol(Phila)2013;51:385–93.
- Masson R, Colas V , Parienti JJ, et al. A comparison of survival with and without extracorporeal life support treatment for severe poisoning due to drug intoxication. Resuscitation 2012; 83:1413–7.
- Proudfoot AT, Krenzelok EP, Vale JA. Position Paper on urine alkalinization. J Toxicol Clin Toxicol2004;42:1–26.
- Greene S, Harris C, Singer J. Gastrointestinal decontamination of the poisoned patient. Pediatr EmergCare2008;24:176–86,quiz187–9.
- Benson BE, Hoppu K, Troutman WG, et al. Position paper update: gastric lavage for gastrointestinal decontamination. Clin Toxicol (Phila)2013;51:140–6.
- Vale JA, Kulig K. Position paper: gastric lavage. J Toxicol Clin Toxicol 2004;42:933–43.
- Chyka PA, Seger D, Krenzelok EP, Vale JA. Position paper: single-dose activated charcoal. Clin Toxicol(Phila)2005;43:61–87.
- Thanacoody R, Caravati EM, Troutman B, et al. Position paper update: whole bowel irrigation for gastrointestinal decontamination of overdose patients. Clin Toxicol(Phila)2015;53:5–12.
- Krenzelok EP. Ipecac syrup-induced emesis. no evidence of benefit. Clin Toxicol (Phila) 2005;43:11–2.
- Position statement and practice guidelines on the use of multi-dose activated charcoal in the treatment of acute poisoning. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol 1999;37:731–51.
- Hojer J, Troutman WG, Hoppu K, et al. Position paper update: ipecac syrup for gastrointestinal decontamination. Clin Toxicol(Phila)2013;51:134–9.
- Skinner CG, Chang AS, Matthews AS, Reedy SJ, Morgan BW. Randomized controlled study on the use of multiple-dose activated charcoal in patients with supratherapeutic phenytoin levels. Clin Toxicol (Phila)2012;50:764–9.
- Brahmi N, Kouraichi N, Thabet H, Amamou M. Influence of activated charcoal on the pharmacokinetics and the clinical features of carbamazepine poisoning. Am J Emerg Med 2006;24:440–3.
- Pitetti RD, Singh S, Pierce MC. Safe and efficacious use of procedural sedation and analgesia by non anesthesiologists in a pediatric emergency department. Arch Pediatr Adolesc Med 2003;157:1090–6.
- Treatment of benzodiazepine overdose with flumazenil. The Flumazenil in Benzodiazepine Intoxication Multicenter Study Group. Clin Ther 1992;14: 978–95.
- Lheureux P, Vranckx M, Leduc D, Askenasi R. Flumazenil in mixed benzodiazepine/tricyclic anti depressant overdose: a placebo-controlled study in the dog. Am J Emerg Med 1992;10:184– 8.
- Beauvoir C, Passeron D, du Cailar G, Millet E. Diltiazem poisoning: hemodynamic aspects. Ann Fr Anesth Reanim 1991;10:154–7.
- Gillart T, Loiseau S, Azarnoush K, Gonzalez D, Guelon D. Resuscitation after three hours of cardiac arrest with severe hypothermia following a toxic coma. Ann Fr Anesth Reanim 2008;27:510–3.
- Nordt SP, Clark RF. Midazolam: a review of therapeutic uses and toxicity. J Emerg Med 1997;15:357–65.
- Machin KL, Caulkett NA. Cardiopulmonary effects of propofol and a medetomidine-midazolam-ketamine combination in mallardducks. Am JVet Res1998;59:598–602.
- Osterwalder JJ. Naloxone – for intoxications with intravenous heroin and heroin mixtures– harmless or hazardous? A prospective clinical study. J Toxicol Clin Toxicol 1996; 34:409–16.
- Sporer KA, Firestone J, Isaacs SM. Out-of-hospital treatment of opioid overdoses in an urban setting. Acad Emerg Med 1996;3:660–7.
- Wanger K, Brough L, Macmillan I, Goulding J, MacPhail I, Christenson JM. Intravenous vs subcutaneous naloxone for out-of-hospital management of presumed opioid overdose. Acad Emerg Med 1998; 5:293–9.
- Hasan RA, Benko AS, Nolan BM, Campe J, Duff J, Zureikat GY . Cardiore spiratory effects of naloxone in children. Ann Pharmac other 2003;37:1587–92.
- Sporer KA. Acute heroin overdose. Ann Intern Med 1999;130:584–90.
- Kaplan JL, Marx JA, Calabro JJ, et al. Double-blind, randomized study of nalmefene and naloxone in emergency department patients with suspected narcotic overdose. Ann Emerg Med 1999;34:42–50.
- Schneir AB, Vadeboncoeur TF, Offerman SR, et al. Massive Oxy Continingestion refractory to naloxone therapy. Ann Emerg Med 2002; 40:425–8.
- Kelly AM, Kerr D, Dietze P, Patrick I, Walker T, Koutsogiannis Z. Randomised trial of intranasal versus intra muscular naloxone in prehospital treatment for suspected opioid overdose. Med J Aust 2005; 182: 24–7.
- Robertson TM, Hendey GW, Stroh G, Shalit M. Intranasal naloxone is a viable alternative to intravenous naloxone for prehospital narcotic overdose. Prehosp Emerg Care 2009;13:512–5.
- Kerr D, Kelly AM, Dietze P, Jolley D, Barger B. Randomized controlled trial comparing the effectiveness and safety of intranasal and intramuscular naloxone for the treatment of suspected heroin overdose. Addiction 2009; 104:2067–74.
- Barton ED, Colwell CB, Wolfe T, et al. Efficacy of intra nasalnaloxoneasa needle less alternative for treatment of opioid overdose in the prehospital setting. J Emerg Med 2005;29:265–71.
- Boyd JJ, Kuisma MJ, Alaspaa AO, Vuori E, Repo JV, Randell TT. Recurrent opioid toxicity after pre-hospital care of presumed heroin overdose patients. Acta Anaesthesiol Scand 2006;50:1266–70.
- Buajordet I, Naess AC, Jacobsen D, Brors O. Adverse events after naloxone treatment of episodes of suspected acute opioid overdose. Eur JEmerg Med 2004;11:19–23.
- Cantwell K, Dietze P, Flander L. The relationship between naloxone dose and key patient variables in the treatment of non-fatal heroin overdose in the prehospital setting. Resuscitation 2005;65:315–9.
- Cetrullo C, Di Nino GF, Melloni C, Pieri C, Zanoni A. Naloxone antagonism toward opiate analgesic drugs. Clinical experimental study. Minerva Anestesiol 1983;49:199–204.
- Nielsen K, Nielsen SL, Siersma V, Rasmussen LS. Treatment of opioid overdose in a physician-based prehospital EMS: frequency and long-termprognosis. Resuscitation2011;82:1410–3.
- Stokland O, Hansen TB, Nilsen JE. Prehospital treatment of heroin intoxication in Oslo in 1996. Tidsskr Nor Laege foren 1998; 118:3144–6.
- Wampler DA, Molina DK, McManus J, Laws P, Manifold CA. Nodeaths associated with patient refusal of transport after naloxone-reversed opioid overdose. Prehosp Emerg Care 2011;15:320–4.
- Tokarski GF, Young MJ. Criteria for admitting patients with tricyclic anti depressant overdose. J Emerg Med 1988;6:121–4.
- Banahan Jr BF, Schelkun PH. Tricyclic anti depressant overdose: conservative management in a community hospital with cost-saving implications. JEmerg Med 1990;8:451–4.
- Hulten BA, Adams R, Askenasi R, et al. Predicting severity of tricyclicanti depressant overdose. J Toxicol Clin Toxicol 1992;30:161–70.
- Bailey B, Buckley NA, Amre DK. A meta-analysis of prognostic indicators to predict seizures, arrhythmias or death after tricyclic antidepressant overdose. J Toxicol Clin Toxicol 2004; 42:877–88.
- Thanacoody HK, Thomas SH. Tricyclic antidepressant poisoning: cardiovascular toxicity. Toxicol Rev 2005; 24:205–14.
- Woolf AD, Erdman AR, Nelson LS, et al. Tricyclic anti depressant poisoning: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol(Phila)2007;45:203–33.
- Hoffman JR, Votey SR, Bayer M, Silver L. Effect of hypertonic sodium bicarbonate in the treatment of moderate-to-severe cyclic antidepressant overdose. Am J Emerg Med 1993;11:336–41.
- Koppel C, Wiegreffe A, Tenczer J. Clinical course, therapy, outcome and analytical data in amitriptyline and combined amitriptyline/chlordiazepoxide overdose. Hum Exp Toxicol 1992;11:458–65.
- Hedges JR, Baker PB, Tasset JJ, Otten EJ, Dalsey WC, Syverud SA. Bicarbonate therapy for the cardiovascular toxicity of amitriptyline in an animal model. J Emerg Med 1985;3:253–60.
- Knudsen K, Abrahamsson J. Epinephrine and sodium bicarbonate independently and additively increase survival in experimental amitriptyline poisoning. Crit Care Med 1997;25:669–74.
- Sasyniuk BI, Jhamandas V , V alois M. Experimental amitriptyline intoxication: treatment of cardiac toxicity with sodium bicarbonate. Ann Emerg Med 1986;15:1052–9.
- Bradberry SM, Thanacoody HK, Watt BE, Thomas SH, Vale JA. Management of the cardiovascular complications of tricyclicanti depressant poisoning: role of sodium bicarbonate. Toxicol Rev 2005;24:195–204.
- YoavG,OdeliaG,ShaltielC. Alipid emulsion reduces mortality from clomipramine overdose in rats. Vet Hum Toxicol 2002;44:30.
- Harvey M, Cave G. Intralipid outperforms sodium bicarbonate in a rabbit model of clomipramine toxicity. Ann Emerg Med 2007;49:178–85,185.e1–4.
- Brunn GJ, Keyler DE, Pond SM, Pentel PR. Reversal of desipramine toxicity in rats using drug-specific antibody Fab fragment: effects on hypotension and interaction with sodium bicarbonate. JPharmacol Exp Ther 1992;260:1392–9.
- Brunn GJ, Keyler DE, Ross CA, Pond SM, Pentel PR. Drug-specific F(ab desipramine cardio toxicity in rats. Int JI mmuno pharmacol 1991;13:841–51. fragment reduces
- Hursting MJ, Opheim KE, Raisys VA, Kenny MA, Metzger G. Tricyclic antidepressant- specific Fab fragments alter the distribution and elimination of desipramine in the rabbit: a model for overdose treatment. J Toxicol Clin Toxicol 1989;27:53–66.
- Pentel PR, Scarlett W, Ross CA, Landon J, Sidki A, Keyler DE. Reduction of desipramine cardio toxicity and prolon gation of survival in rats with the use of polyclonal drug-specific antibody Fabf ragments. Ann Emerg Med 1995;26:334–41.
- Pentel PR, Ross CA, Landon J, Sidki A, Shelver WL, Keyler DE. Reversal of desipramine toxicity in rats with polyclonal drug-specific antibody Fab fragments. J Lab Clin Med 1994;123:387–93.
- Dart RC, Sidki A, Sullivan Jr JB, Egen NB, Garcia RA. Ovine desipramine antibody fragments reverse desipramine cardio vascular toxicity in the rat.AnnEmerg Med1996;27:309–15.
- Heard K, Dart RC, Bogdan G, et al. A preliminary study of tricyclic antidepressant (TCA) ovine FAB for TCA toxicity. Clin Toxicol(Phila) 2006;44:275–81.
- Pentel P, Peterson CD. Asystole complicating physostigmine treatment of tricyclic antidepressant overdose. Ann Emerg Med 1980;9:588–90.
- Lange RA, Cigarroa RG, Yancy Jr CW, et al. Cocaine-induced coronary-artery vasoconstriction. NEngl J Med 1989;321:1557–62.
- aumann BM, Perrone J, Hornig SE, Shofer FS, Hollander JE. Randomized, double-blind, placebo-controlled trial of diazepam, nitroglycerin, or both for treatment of patients with potential cocaine-associated acute coronary syndromes. Acad Emerg Med 2000;7:878–85.
- Honderick T, Williams D, Seaberg D, Wears R. A prospective, randomized, controlled trial of benzodiazepines and nitroglycerine or nitroglycerine alone in the treatment of cocaine-associated acute coronary syndromes. AmJEmerg Med2003;21:39–42.
- Negus BH, Willard JE, Hillis LD, et al. Alleviation of cocaine-induced coronary vasoconstriction within travenous verapamil. Am J Cardiol 1994;73: 510–3.
- Saland KE, Hillis LD, Lange RA, Cigarroa JE. Influence of morphine sulfate on cocaine-induced coronary vasoconstriction. Am J Cardiol 2002;90:810–1.
- Brogan WCI, Lange RA, Kim AS, Moliterno DJ, Hillis LD. Alleviation of cocaine-induced coronary vasoconstriction by nitroglycerin. JAm Coll Cardiol 1991;18:581–6.
- Hollander JE, Hoffman RS, Gennis P, et al. Nitroglycerinin the treatment of cocaine associated chest pain–clinical safety and efficacy. J Toxicol Clin Toxicol 1994;32:243–56.
- Dattilo PB, Hailpern SM, Fearon K, Sohal D, Nordin C. Beta-blockers are associated with reduced risk of myocardial infarction after cocaine use. Ann Emerg Med 2008;51:117–25.
- Vongpatanasin W, Mansour Y, Chavoshan B, Arbique D, Victor RG. Cocaine stimulates the human cardiovascular system via a central mechanism of action. Circulation1999;100:497–502.
- Lange RA, Cigarroa RG, Flores ED, et al. Potentiation of cocaine-induced coronary vasoconstriction by beta-adrenergic blockade. Ann Intern Med 1990;112:897–903.
- Sand IC, Brody SL, Wrenn KD, Slovis CM. Experience with esmolol for the treatment of cocaine-associated cardiovascular complications. Am J Emerg Med 1991;9:161–3.
- Sofuoglu M,Brown S, Babb DA, Pentel PR, Hatsukami DK. Carvedilol affects the physiological and behavioral response to smoked cocaine in humans. Drug Alcohol Depend 2000;60:69–76.
- Sofuoglu M, Brown S, Babb DA, Pentel PR, Hatsukami DK. Effects of labetalol treatmentonthe physiological and subjective response to smoked cocaine. Pharmacol Biochem Behav 2000;65:255–9.
- Boehrer JD, Moliterno DJ, Willard JE, Hillis LD, Lange RA. Influence of labetalol on cocaine-induced coronary vasoconstriction in humans. Am J Med 1993;94:608–10.
- Hsue PY , McManus D, Selby V , et al. Cardiac arrest in patients who smoke crack cocaine. Am J Cardiol 2007;99:822–4.
- Litz RJ, Popp M, Stehr SN, Koch T. Successful resuscitation of a patient with ropivacaine-induced asystole after axillary plexus block using lipid infusion. Anaesthesia 2006;61:800–1. 339.
- Rosenblatt MA, Abel M, Fischer GW, Itzkovich CJ, Eisenkraft JB. Successful use of a 20% lipid emulsion to resuscitate a patient after a presumed bupi vacaine related cardiac arrest. Anesthesiology 2006; 105:217–8.
- Marwick PC, Levin AI, Coetzee AR. Recurrence of cardiotoxicity after lipid rescue from bupi vacaine-induced cardiac arrest. Anesth Analg 2009;108:1344–6.
- Warren JA, Thoma RB, Georgescu A, Shah SJ. Intravenous lipid infusion in the successful resuscitation of local anesthetic-induced cardiovascular collapse after supraclavicular brachial plexusblock. Anesth Analg 2008; 106:1578–80, table of contents.
- Smith HM, Jacob AK, Segura LG, Dilger JA, Torsher LC. Simulation education in anesthesia training: a case report of successful resuscitation of bupivacaine induced cardiac arrest linked to recent simulation training. Anesth Analg 2008;106:1581–4, table of contents.
- Foxall GL, Hardman JG, Bedforth NM. Three-dimensional, multiplanar, ultrasound-guided, radial nerve block. Reg Anesth Pain Med 2007;32:516–21.
- Shah S, Gopalakrishnan S, Apuya J, Martin T. Use of Intralipid in an infant with impending cardiovascular collapse due to local anesthetic toxicity. J Anesth 2009;23:439–41.
- Zimmer C, Piepenbrink K, Riest G, Peters J. Cardiotoxic and neurotoxic effects after accidental intravascular bupi vacaine administration. Therapy with lidocaine propofol and lipid emulsion. Der Anaesthesist 2007; 56:449–53.
- Litz RJ, Roessel T, Heller AR, Stehr SN. Reversal of central nervous system and cardiac toxicity after local anesthetic intoxication by lipid emulsion injection. Anesth Analg 2008;106:1575–7, table of contents.
- Ludot H, Tharin JY , Belouadah M, Mazoit JX, Malinovsky JM. Successful resuscitation after ropi vacaine and lidocaine-induced ventricular arrhythmia following posterior lumbar plexus block in a child. Anesth Analg 2008; 106:1572–4.
- Cave G, Harvey MG, Winterbottom T. Evaluation of the Association of Anaesthetists of Great Britain and Ireland lipid infusion protocol in bupivacaine induced cardiac arrest in rabbits. Anaesthesia2009;64:732–7.
- Di Gregorio G, Schwartz D, Ripper R, et al. Lipid emulsion is superior to vasopressin in a rodent model of resuscitation from toxin-induced cardiac arrest. Crit Care Med 2009;37:993–9.
- Weinberg GL, Vade Boncouer T, Ramaraju GA, Garcia-Amaro MF,Cwik MJ.Pretreatmentor resuscitation with a lipid infusion shifts the dose response to bupi vacaine-induced asystole in rats. Anesthesiology 1998; 88: 1071–5.
- Weinberg G, Ripper R, Feinstein DL, Hoffman W. Lipid emulsion infusion rescues dogs from bupi vacaine-induced cardiac toxicity. Reg Anesth Pain Med 2003;28:198–202.
- Weinberg GL, DiGregorio G, Ripper R, et al. Resuscitation with lipid versus epinephrine in a rat model of bupi vacaine overdose. Anesthesiology 2008;108:907–13.
- Barton CA, Johnson NB, Mah ND, Beauchamp G, Hendrickson R. Successful treatment of a massive metoprolol overdose using intravenous lipid emulsion and hyperinsulinemia/euglycemiatherapy. Pharmacotherapy 2015;35:e56–60.
- Management of Severe Local Anaesthetic Toxicity. Association of Anaesthetists of Great Britain and Ireland; 2010 [accessed28.06.10].
- Hicks SD, Salcido DD, Logue ES, et al. Lipid emulsion combined with epinephrine and vasopressin does not improve survival in a swine model of bupi vacaine-induced cardiac arrest. Anesthesiology 2009;111:138–46.
- Hiller DB, Gregorio GD, Ripper R, et al. Epinephrine impairs lipid resuscitation from bupi vacaine overdose: a threshold effect. Anesthesiology 2009;111:498–505. Bailey B. Glucagon in beta-blocker and calcium channel blocker overdoses: a systematic review. J Toxicol Clin Toxicol 2003;41:595–602.
- Bailey B. Glucagon in beta-blocker and calcium channel blocker overdoses: a systematic review. J Toxicol Clin Toxicol 2003;41:595–602.
- Fahed S, Grum DF, Papadimos TJ. Labetalol infusion for refractory hypertension causing severe hypotension and brady cardia: an issue of patient safety.Patient Saf Surg 2008;2:13.
- Fernandes CM, Daya MR. Sotalol-induced bradycardia reversed by glucagon. Can Fam Physician 1995; 41:659–60,663–5.
- Frishman W, Jacob H, Eisenberg E, Ribner H. Clinical pharmacology of the new beta-adrenergic blocking drugs. Part 8. Self-poisoning with beta-ad renoceptor blocking agents: recognition and management. Am Heart J1979;98: 798–811.
- Gabry AL, Pourriat JL, Hoang TD, Lapandry C. Cardiogenic shock caused by metoprolol poisoning. Reversibility with high doses of glucagon and isoproterenol. Presse Med 1985;14:229.
- Hazouard E, Ferrandiere M, Lesire V, Joye F, Perrotin D, de Toffol B. Peduncular hallucinosis related to propranolol self-poisoning: efficacy of intravenous glucagon. Intensive Care Med 1999;25:336–7.
- Khan MI, Miller MT. Beta-blocker toxicity–the role of glucagon. Report of 2 cases. SAfr Med J 1985;67:1062–3.
- Moller BH. Letter: massive intoxication with metoprolol. BrMedJ 1976;1:222.
- O’Mahony D,O’Leary P, Molloy MG. Severe oxprenolol poisoning: the importance of glucagon infusion. HumExpToxicol1990;9:101–3.
- Wallin CJ, Hulting J. Massive metoprolol poisoning treated with prenalterol. Acta Med Scand 1983;214:253–5.
- Weinstein RS, Cole S, Knaster HB, Dahlbert T. Betablocker overdose. with propranololand with atenolol. Ann Emerg Med1985;14:161–3.
- Alderfliegel F, Leeman M, Demaeyer P, Kahn RJ. Sotalol poisoning associated with asystole. IntensiveCareMed1993;19:57–8.
- Kenyon CJ, Aldinger GE, Joshipura P , Zaid GJ. Successful resuscitation using external cardiac pacing in beta adrenergic antagonist-induced bradyasystolic arrest. Ann Emerg Med 1988;17:711–3.
- Freestone S, Thomas HM, Bhamra RK, Dyson EH. Severe atenolol poisoning: treatment with prenalterol. Hum Toxicol 1986;5:343–5.
- Kerns W, Schroeder II, Williams D, Tomaszewski C, Raymond CR.Insulin improves survival in acanine model of acute beta-blocker toxicity. Ann Emerg Med1997;29:748–57.
- Holger JS, Engebretsen KM, Fritzlar SJ, Patten LC, Harris CR, Flottemesch TJ. Insulin versus vasopressin and epinephrine to treat beta-blocker toxicity. Clin Toxicol (Phila) 2007;45:396– 401.
- Page C, Hacket LP, Isbister GK. The use of high-dose insulin-glucose euglycemia blocke roverdose: a case report. J Med Toxicol 2009;5:139–43.
- Jovic-Stosic J, Gligic B, Putic V, Brajkovic G, Spasic R. Severe propranolol and ethanol overdose with wide complex tachycardia treated with intravenous lipid emulsion: a case report. ClinToxicol (Phila)2011;49:426–30.
- Barton CA, Johnson NB, Mah ND, Beauchamp G, Hendrickson R. Successful treatment of a massive metoprolol overdose using intravenous lipid emulsion and hyperinsulinemia/euglycemiatherapy. Pharmaco therapy 2015;35:e56–60.
- Sebe A, Disel NR, Acikalin Akpinar A, Karakoc E. Role of intravenous lipid emulsions in the management of calcium channel blocker and beta blocker overdose: 3 years experience of a university hospital.Postgrad Med 2015;127:119–24.
- Doepker B, Healy W, Cortez E, Adkins EJ. High-dose insulin and intravenous lipid emulsion therapy for cardio genic shock induced by intentional calcium channel blocker and beta-blocker overdose: a case series. J Emerg Med 2014;46:486–90.
- Kollef MH. Labetalol overdose successfully treated with amrin one and alpha adrenergic receptor agonists. Chest 1994;105:626–7.
- O’Grady J, Anderson S, Pringle D. Successful treatment of severe atenolol overdose with calcium chloride. CJEM 2001;3: 224–7.
- McVey FK, Corke CF. Extracorporeal circulation in the management of massive propranolol overdose.Anaesthesia 1991;46:744–6.
- Lane AS, Woodward AC, Goldman MR. Massive propranolol overdose poorly responsive to pharmacologic therapy: use of the intra-aorticballo on pump. Ann Emerg Med 1987;16:1381–3.
- Rooney M, Massey KL, Jamali F, Rosin M, Thomson D, Johnson DH. Acebutolol overdose treated with hemodialysis and extracorporeal membrane oxygenation. J Clin Pharmacol 1996;36:760–3.
- Brimacombe JR, Scully M, Swainston R. Propranolol overdose–a dramatic response to calcium chloride. Med J Aust 1991; 155:267–8.
- Bronstein AC, Spyker DA, Cantilena Jr LR, Green JL, Rumack BH, Giffin SL. 2008 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS):26 th annual report. ClinToxicol(Phila) 2009;47:911–1084.
- Olson KR, Erdman AR, Woolf AD, et al. Calcium channel blocker ingestion: an evidence-based consensus guideline for out-of-hospital management. Clin Toxicol (Phila) 2005;43:797–822.
- St-Onge M, Dube PA, Gosselin S, et al. Treatment for calcium channel blocker poisoning: a systematic review.ClinToxicol(Phila)2014;52:926–44.
- Levine M, Curry SC, Padilla-Jones A, Ruha AM. Critical care management of verapamil and diltiazem overdose with a focus on vasopressors: a 25-year experience at a single center. Ann Emerg Med 2013;62:252–8.
- Cohen V, Jellinek SP, Fancher L, et al. Tarka(R) (trandol april/verapamil hydrochloride extended-release) overdose. J Emerg Med 2011;40:291–5.
- Greene SL, Gawarammana I, Wood DM, Jones AL, Dargan PI. Relative safety of hyperinsulinaemia/euglycaemia therapy in the management of calcium channel blocker overdose: a prospective observational study. Intensive Care Med 2007;33:2019–24.
- Harris NS. Case records of the Massachusetts General Hospital. Case 24-2006. A 40-year-old woman with hypotension after an overdose of amlodipine. N Engl J Med 2006;355:602–11.
- Johansen KK, Belhage B. A 48-year-old woman’s survival from a massive verapamil overdose. Uge skr Laeger 2007;169:4074–5.
- Kanagarajan K, Marraffa JM, Bouchard NC, Krishnan P, Hoffman RS, Stork CM. The use of vasopressin in the setting of recalcitrant hypotension due to calcium channel blocker overdose. Clin Toxicol (Phila) 2007;45:56–9.
- Marques M, Gomes E, de Oliveira J. Treatment of calcium channel blocker intoxication with insulin infusion: case report and literature review. Resuscitation 2003;57:211–3.
- Meyer M, Stremski E, Scanlon M. Successful resuscitation of a verapamil intoxicated child with adextrose-insulin infusion. Clin Intensive Care 2003;14:109–13.
- Ortiz-Munoz L, Rodriguez-Ospina LF, Figueroa-Gonzalez M. Hyper insulin emiceu glycemic therapy for intoxication with calcium channel blockers. BolAsoc MedPR2005;97:182–9.
- Patel NP, Pugh ME, Goldberg S, Eiger G. Hyper insuline mice uglycemia therapy for verapamil poisoning: case report. Am J Crit Care 2007;16:18–9.
- Rasmussen L, Husted SE, Johnsen SP. Severe intoxication after an intentional overdose of amlodipine. Acta Anaesthesiol Scand 2003; 47:1038–40.
- Smith SW, Ferguson KL, Hoffman RS, Nelson LS, Greller HA. Prolonged severe hypotension following combined amlodipine and valsartaningestion. Clin Toxicol (Phila) 2008;46:470–4.
- Eddleston M, Rajapakse S, Rajakanthan, et al. Anti-digoxin Fab fragments in cardio toxicity induced by ingestion of yellow oleander: a randomised controlled trial. Lancet 2000;355:967–model of bupi vacaine overdose. Anesthesiology 2008;108:907–13.
- Lapostolle F, Borron SW, Verdier C, et al. Digoxin-specific Fab fragments as single first-line therapy in digital is poisoning. Crit Care Med 2008;36:3014–8.
- Chan BS, Buckley NA. Digoxin-specific antibody fragments in the treatment of digoxin toxicity. ClinToxicol(Phila)2014;52:824–36.
- Dasgupta A, Szelei-Stevens KA. Neutralization of free digoxin-like immunoreactive components of oriental medicines Dan Shen and Lu-Shen-Wan by the Fab fragment of antidigoxin antibody(Digibind).AmJClinPathol 2004;121:276–81.
- Bosse GM, Pope TM. Recurrent digoxin overdose and treatment with digoxin specific Fab antibody fragments. JEmerg Med 1994;12:179–85.
- Borron SW, Baud FJ, Barriot P, Imbert M, Bismuth C. Prospective study of hydroxocobalamin for acute cyanide poisoning in smoke inhalation. Ann Emerg Med 2007; 49:794–801,801.e1–2.
- Espinoza OB, Perez M, Ramirez MS. Bittercassava poisoning in eight children: a case report.Vet Hum Toxicol 1992;34:65.
- Houeto P, Hoffman JR, Imbert M, Levillain P, Baud FJ. Relation of blood cyanide to plasma cyanocobalamin concentration after a fixed dose of hydroxocobalamin in cyanide poisoning. Lancet 1995; 346:605–8.
- Pontal P, Bismuth C, Garnier R. Therapeutic attitude in cyanide poisoning: retrospective study of 24 non-lethal cases. Vet Hum Toxicol 1982;24:286–7.
- Kirk MA, Gerace R, Kulig KW. Cyanide and methemoglobin kinetics in smoke inhalation victims treated with the cyanide antidotekit. Ann Emerg Med 1993;22:1413–8.
- Chen KK, Rose CL. Nitrite and thiosulfate therapy in cyanide poisoning. J Am Med Assoc 1952;149:113–9.
- Yen D, Tsai J, Wang LM, et al. The clinical experience of acute cyanide poisoning. Am J Emerg Med 1995; 13:524–8.
- Reade MC, Davies SR, Morley PT, Dennett J, Jacobs IC, Australian Resuscitation Council. Review article: management of cyanide poisoning. EmergMed Australas 2012;24:225–38.
- StreitzMJ,BebartaVS,BorysDJ,MorganDL.Patternsofcyanideantidoteuse sinceregulatory AmJTher 2014;21:244–9.
- DriesDJ,EndorfFW.Inhalationinjury:epidemiology,pathology,treatment ScandJ TraumaResuscEmergMed2013;21:31.
- Iqbal S, ClowerJH,BoehmerTK,YipFY,Garbe P. Carbonmonoxide-related hospitalizations S.:evaluationofaweb-basedquerysystemforpublic healthsurveillance.PublicHealth Rep2010;125:423–32.
- Hampson NB, Hauff Carboxyhemoglobinlevels in carbon monoxide poisoning: do they correlatewiththeclinicalpicture?AmJEmergMed 2008;26:665–9.
- Buckley NA, Juurlink DN, Isbister G, Bennett MH,Lavonas Hyperbaricoxygen forcarbon monoxidepoisoning.CochraneDatabaseSystRev2011:CD002041.
- WeaverLK,Clinicalpractice.Carbonmonoxidepoisoning.NEnglJMed 2009;360:1217–25.
- JuurlinkDN,BuckleyNA,StanbrookMB,IsbisterGK,BennettM,McGuiganMA. Hyperbaric CochraneDatabaseSyst Rev2005:CD002041.
- Roderique JD, Josef CS, Feldman MJ, Spiess BD. A modern literature review of carbon monoxide poisoning theories, therapies, and potential targets for therapy advancement. Toxicology2015;334:45–58.
- Satran D, Henry CR, Adkinson C, Nicholson CI, Bracha Y, Henry TD. Cardiovascular manifestations of moderate to severe carbon monoxide poisoning. J Am Coll Cardiol 2005;45:1513–6.
- Henry CR, Satran D, Lindgren B, Adkinson C, Nicholson CI, Myocardial injuryand long-term mortality following moderate to severe carbon monoxide poisoning. JAMA 2006;295:398–402.
- Braz LG, Modolo NS, do Nascimento Jr P, et al. Perioperativecardiacarrest: a study of 53,718 BrJ Anaesth2006;96:569–75.
- Sprung J, Warner ME, Contreras MG, et al. Predictors of survival following cardiac arrest in patientsundergoingnoncardiacsurgery: a studyof 518,294 Anesthesiology2003;99:259–69.
- Nunnally ME, O’Connor MF, Kordylewski H, Westlake B, Dutton RP. The incidence and risk factors for perioperative cardiac arrest observed in the national anesthesia clinical outcomes AnesthAnalg2015;120:364–70.
- Nunes JC, Braz JR, Oliveira TS, de Carvalho LR, Castiglia YM, Braz LG. Intraoperative and anesthesia-relatedcardiacarrestanditsmortalityinolderpatients: a15-yearsurveyinatertiaryteachinghospital.PLOSONE2014;9:e104041.
- Gonzalez LP, Braz JR, Modolo MP, de Carvalho LR, Modolo NS, Braz LG. Pediatric perioperativecardiacarrestandmortality: astudyfromatertiaryteaching Pediatr Crit CareMed2014;15:878–84.
- Ellis SJ, Newland MC, Simonson JA, et al. Anesthesia-related cardiac arrest. Anesthesiology 2014;120:829–38.
- Newland MC, Ellis SJ, Lydiatt CA, et al. Anesthetic-related cardiac arrest and its mortality: a report covering 72,959 anesthetics over 10 yearsfrom a US Anesthesiology 2002;97:108–15.
- Bhananker SM, Ramamoorthy C, Geiduschek JM, et Anesthesia-related cardiac arrest in children: update from the Pediatric Perioperative Cardiac Arrest Registry. Anesth Analg 2007;105:344–50.
- KrishnaRamachandran S, Mhyre J, Kheterpal S, Predictorsofsurvival fromperioperative cardiopulmonary arrests: a retrospective analysis of 2,524 events from the Get With The Guidelines-Resuscitationregistry.Anesthesiology2013;119:1322–39.
- Brown J, Rogers J, Soar Cardiac arrest during surgery and ventilation in theproneposition: a casereportandsystematicreview.Resuscitation 2001;50:233–8.
- Atkinson MC. The efficacy of cardiopulmonary resuscitation in the prone position. Crit Care Resusc2000;2:188–90.
- MertesPM,TajimaK,Regnier-KimmounMA,etal.Perioperativeanaphylaxis. MedClinNorth Am2010;94:761–89,xi.
- Wolfe JW, Butterworth JF. Local anesthetic systemic toxicity: update on mechanisms and CurrOpinAnaesthesiol2011;24:561–6.
- CaoD,HeardK,ForanM,KoyfmanA.Intravenouslipidemulsionintheemergencydepartment: JEmergMed 2015;48:387–97.
- Waring WS. Intravenouslipidadministrationfordrug-inducedtoxicity: a criticalreviewofthe ExpertRevClinPharmacol2012;5: 437–44.
- Neal JM, Mulroy MF, Weinberg GL, American Society of Regional Anesthesia and Pain AmericanSocietyofRegionalAnesthesiaandPainMedicine checklistformanaging localanestheticsystemictoxicity,2012version.Reg AnesthPainMed2012;37:16–8.
- Mazer SP, Weisfeldt M, Bai D, et Reverse CPR: a pilot study of CPR in the prone position. Resuscitation2003;57:279–85.
- MeaneyPA,BobrowBJ,ManciniME, etal.Cardiopulmonaryresuscitationquality:improving cardiacresuscitationoutcomesbothinsideandoutsidethe hospital:aconsensusstatementfrom Circulation2013;128:417–35.
- Martin GB, Carden DL, Nowak RM, Lewinter JR, Johnston W, Tomlanovich MC. Aortic and right atrial pressures during standard and simultaneous compression and ventilation CPR in AnnEmergMed1986;15:125–30.
- TimermanS,CardosoLF,RamiresJA,HalperinH.Improvedhemodynamic performancewitha novel chest compression device during treatment of inhospital cardiac Resuscitation 2004;61:273–80.
- Niemann JT, Rosborough JP, Ung S, Criley JM. Coronary perfusion pressure during AnnEmergMed 1982;11:127–31.
- Nolan JP, Neumar RW, Adrie C, et Post-cardiac arrest syndrome: epidemiology pathophysiology treatment and prognostication: a scientific statement from the International
- Siriphuwanun V, Punjasawadwong Y, Lapisatepun W, Charuluxananan S, Uerpairojkit K. Incidence of and factors associated with perioperative cardiac arrest within 24 hours of RiskManagHealthcPolicy2014;7:155–62. Liaison Committee on Resuscitation; the American Heart Association Emergency Cardiovascular Care Committee the Council on Cardiovascular Surgery and Anesthesia; the CouncilonCardiopulmonaryPerioperativeandCriticalCaretheCouncilonClinicalCardiology theCouncilon Stroke.Resuscitation2008;79:350–79.
- British Medical Association the Resuscitation Council (UK), Royal College of Decisionsrelatingtocardiopulmonaryresuscitation. Ajointstatment fromtheBritishMedical Association,theResuscitationCouncil(UK)andthe RoyalCollegeofNursing.London:British MedicalAssociation;2014.
- Charalambous CP, Zipitis CS, Keenan DJ. Chest reexploration in the intensive care unit after cardiac surgery: a safe alternative to returning to the operating Ann Thorac Surg 2006;81:191–4.
- McKowen RL, Magovern GJ, Liebler GA, Park SB, Burkholder JA, Maher Infectious complications and cost-effectiveness of open resuscitation in the surgical intensive care unit aftercardiacsurgery.AnnThoracSurg1985;40: 388–92.
- Pottle A, Bullock I, Thomas J, Scott L. Survival to discharge following Open Chest Cardiac Compression(OCCC).A4-yearretrospectiveauditinacardiothoracic specialistcentre–Royal BromptonandHarefieldNHSTrust,UnitedKingdom. Resuscitation2002;52:269–72.
- Mackay JH, Powell SJ, Osgathorp J, Rozario CJ. Six-year prospectiveauditof chest reopening EurJCardiothoracSurg2002;22:421–5.
- Birdi I, Chaudhuri N, Lenthall K, Reddy S, Nashef Emergency reinstitution of cardiopulmonary bypass following cardiac surgery: outcome justifies the cost. Eur J CardiothoracSurg2000;17:743–6.
- el-BanayosyA,BrehmC,KiznerL,etal.Cardiopulmonaryresuscitationafter cardiacsurgery:a two-yearstudy.JCardiothoracVascAnesth1998;12:390–2.
- Anthi A, Tzelepis GE, Alivizatos P, Michalis A, Palatianos GM, Geroulanos Unexpected cardiacarrestaftercardiacsurgery: incidence, predisposing causes, andoutcomeofopenchest cardiopulmonaryresuscitation.Chest 1998;113:15–9.
- Wahba A, Gotz W, Birnbaum Outcome of cardiopulmonaryresuscitation following open heartsurgery.ScandCardiovascJ1997;31:147–9.
- Kaiser GC, Naunheim KS, Fiore AC, et al. Reoperation in the intensive care unit. Ann Thorac Surg1990;49:903–7,discussion908.
- LaPar DJ, Ghanta RK, Kern JA, et al. Hospital variation in mortality from cardiac arrest after cardiacsurgery:anopportunityforimprovement?AnnThoracSurg 2014;98:534–9,discussion 539–40.
- RhodesJF,BlaufoxAD,SeidenHS,etal.Cardiacarrestininfantsaftercongenital Circulation1999;100:II194–9.
- Kempen PM, Allgood R. Right ventricular rupture during closed-chest cardiopulmonary resuscitation after pneumonectomy with pericardiotomy: a case report. Crit Care Med 1999;27:1378–9.
- Bohrer H, Gust R, Bottiger BW. Cardiopulmonary resuscitation after cardiac J CardiothoracVascAnesth1995;9:352.
- Klintschar M, Darok M, Radner H. Massive injury to the heart after attempted active compression–decompressioncardiopulmonaryresuscitation.IntJLegal Med1998;111:93–6.
- Fosse E, Lindberg H. Left ventricular rupture following external chest compression. Acta AnaesthesiolScand1996;40:502–4.
- Li Y, Wang H, Cho JH, et Defibrillationdeliveredduringtheupstroke phaseofmanualchest compressionimprovesshocksuccess.CritCareMed 2010;38:910–5.
- Li Y, Yu T, Ristagno G, et al. The optimalphasicrelationshipbetween synchronized shock and Resuscitation 2010;81:724–9.
- LarsenAI,HjornevikAS,EllingsenCL,NilsenDW.Cardiacarrestwith continuousmechanical chestcompressionduringpercutaneouscoronary intervention.AreportontheuseoftheLUCAS Resuscitation 2007;75:454–9.
- Tsao NW, Shih CM, Yeh JS, et al. Extracorporeal membrane oxygenationassisted primary percutaneous coronary intervention may improve survival of patients with acute myocardial JCritCare2012;27,530.e1–11.
- Alpert MA. Sudden cardiac arrest and sudden cardiac death on dialysis: epidemiology, evaluation,treatment,andprevention.HemodialInt 2011;15:S22–9.
- Sacchetti A, Stuccio N, Panebianco P, Torres EDhemodialysisfortreatment ofrenalfailure emergencies.AmJEmergMed1999;17:305–7.
- PutchaN,AllonM.Managementofhyperkalemiaindialysispatients.Semin Dial2007;20:431–
- Bleyer AJ, Russell GB, Satko Sudden and cardiac death rates in hemodialysis patients. KidneyInt1999;55:1553–9.
- AlfonzoAV,SimpsonK,DeighanC,CampbellS,FoxJ.Modificationstoadvanced lifesupport Resuscitation2007;73:12–28.
- DavisTR,YoungBA,EisenbergMS,ReaTD,CopassMK,CobbLA.Outcomeof cardiacarrests attendedbyemergencymedicalservicesstaffatcommunity Kidney Int2008;73:933–9.
- Lafrance JP, Nolin L, Senecal L, Leblanc M. Predictors and outcome of cardiopulmonary resuscitation (CPR) calls in a large haemodialysis unit over a seven-year Nephrol Dial Transplant2006;21:1006–12.
- MeaneyPA,NadkarniVM,Kern KB,IndikJH,Halperin HR,Berg RA.Rhythms andoutcomes ofadultin-hospitalcardiacarrest.CritCareMed2010;38:101–8.
- GirotraS,NallamothuBK,SpertusJA,etal.Trendsinsurvivalafterin-hospital N EnglJMed2012;367:1912–20.
- Bird S, Petley GW, Deakin CD, Clewlow F. Defibrillationduringrenaldialysis: a survey of UK Resuscitation 2007;73:347–53.
- Lehrich RW, Pun PH, Tanenbaum ND, Smith SR, Middleton JP. Automated external defibrillators and survival from cardiac arrest in the outpatient hemodialysis clinic. J Am Soc Nephrol2007;18:312–20.
- Arsati F, Montalli VA, FlorioFM, etal.Braziliandentists’attitudesaboutmedical emergencies JDentEduc2010;74:661–6.
- Girdler NM, Smith Prevalence of emergency events in British dental practice and emergencymanagementskillsofBritishdentists.Resuscitation 1999;41:159–67.
- Qualitystandardsforcardiopulmonaryresuscitationpracticeandtraining.
Primarydentalcare– Quality standards for CPR and training; 2013. Available from: https://www.resus.org.uk/quality-standards/primary-dental-carequality-standards-for-cpr- and-training/.
- Muller MP, Hansel M, Stehr SN, Weber S, Koch T. A state-wide survey of medical emergency managementindentalpractices:incidenceofemergenciesand EmergMed J2008;25:296–300.
- Meechan JG, Skelly Problems complicating dental treatment with local anaesthesia or sedation:preventionandmanagement.DentUpdate 1997;24:278–83.
- JowettNI,CabotLB.Patientswithcardiacdisease:considerationsforthedental Br DentJ2000;189:297–302.
- ChapmanPJ,PenkeymanHW.Successfuldefibrillationofadentalpatientin cardiacarrest.Aust DentJ2002;47:176–7.
- AbsiEG. Acardiacarrestinthedentalchair.BrDentJ1987;163:199–200.
- FujinoH,YokoyamaT,YoshidaK,SuwaK.Usingastoolforstabilizationofa dentalchairwhen Resuscitation2010;81:502.
- LaurentF,SegalN,AugustinP.Chestcompression:notaseffectiveondental chairasonthefloor. Resuscitation2010;81:1729,authorreply1730.
- Lepere AJ, Finn J, Jacobs I. Efficacy of cardiopulmonary resuscitation performed in a dental AustDentJ2003;48:244–7.
- Yokoyama T, Yoshida K, Suwa K. Efficacy of external cardiac compression in a dental chair. Resuscitation2008;79:175–6.
- Segal N, Laurent F, Maman L, Plaisance P, Augustin Accuracy of a feedback device for cardiopulmonaryresuscitationonadentalchair.EmergMedJ 2012;29:890–3.
- Perkins GD, Stephenson BT, Smith CM, Gao A comparison between over-the-head and standardcardiopulmonaryresuscitation.Resuscitation 2004;61:155–61.
- Handley AJ, Handley JA. Performing chest compressions in a confined space. Resuscitation 2004;61:55–61.
- Maisch S, Issleib M, Kuhls B, et al. A comparison between over-the-head and standard cardiopulmonary resuscitation performed by two rescuers: a simulation study. J Emerg Med 2010;39:369–76.
- ChiCH,TsouJY,SuFC.Comparisonofchestcompressionkinematicsassociated withover-the- AmJEmerg Med2009;27:1112–6.
- Perkins GD, Handley AJ, Koster KW, et al. European Resuscitation Council Guidelines for Resuscitation 2015 Section 2. Adult basic life support and automated external defibrillation. Resuscitation2015;95:81–98.
- Rosenberg M. Preparing for medical emergencies: the essential drugs and equipment for the JAmDentAssoc2010;141:14S–9S.
- Resuscitation Council(UK). Qualitystandardsforcardiopulmonaryresuscitationpractice and Acutecare.London:ResuscitationCouncil(UK); 2013.
- HunterPL.Cardiacarrestinthedentalsurgery.BrDentJ1991;170:284.
- Deakin CD, Fothergill R, Moore F, Watson L, Whitbread M. Level of consciousness on admissiontoa HeartAttackCentreisapredictorofsurvivalfrom out-of-hospitalcardiacarrest. Resuscitation2014;85:905–9.
- LaurentF,AugustinP,ZakC,MamanL,SegalN.Preparednessofdental practicestotreatcardiac arrest:availabilityofdefibrillators.Resuscitation 2011;82:1468–9.
- KandrayDP,PierenJA,BennerRW.AttitudesofOhiodentistsanddentalhygienistsontheuseof JDentEduc 2007;71:480–6.
- Safesedationpracticeforhealthcareprocedures:standardsandguidance;2013.Availablefrom: http://www.aomrc.org.uk/docdetails/9737-safesedation-practice-for-healthcare- procedures-standards-and-guidance. competence in resuscitation and occurrence of resuscitation emergencies. Aust Dent J 1995;40:98–103.
- Chapman PJ. A questionnaire survey of dentists regarding knowledge and perceived competence in resuscitation and occurrence of resuscitation emergencies. Aust Dent J 1995;40:98–103.
- Chate Evaluation of a dental practice cardiopulmonary resuscitation training scheme. Br DentJ1996;181:416–20.
- Atherton GJ, Pemberton MN, Thornhill Medicalemergencies: the experience of staff of a UKdentalteachinghospital.BrDentJ2000;188:320–4.
- Sand M, Bechara FG, Sand D, Mann Surgical and medical emergencies on board European aircraft:aretrospectivestudyof10189cases.CritCare 2009;13:R3.
- GrafJ,StubenU,PumpS.In-flightmedicalemergencies.DtschArzteblInt 2012;109:591–601,
- Weinlich M, Nieuwkamp N, Stueben U, Marzi I, Walcher Telemedicalassistanceforin-flight emergenciesonintercontinentalcommercialaircraft.J TelemedTelecare2009;15:409–13.
- Peterson DC, Martin-Gill C, Guyette FX, et Outcomes of medical emergencies on commercialairlineflights.NEnglJMed2013;368:2075–83.
- McLoughlin DC, Jenkins DI. Aircrew periodic medical examinations. Occup Med (Lond) 2003;53:11–4.
- Hung KK, Cocks RA, Poon WK, Chan EY, Rainer TH, Graham Medical volunteers in commercialflightmedicaldiversions.AviatSpaceEnvironMed 2013;84:491–7.
- Valani R, Cornacchia M, Kube Flightdiversions due to onboardmedical emergencies on an internationalcommercialairline.AviatSpaceEnvironMed 2010;81:1037–40.
- O’Rourke MF, Donaldson E, Geddes An airline cardiac arrest program. Circulation 1997;96:2849–53.
- PageRL,JoglarJA,KowalRC,etal.Useofautomatedexternaldefibrillatorsby S.airline.N EnglJMed2000;343:1210–6.
- Brown AM, Rittenberger JC, Ammon CM, Harrington S, Guyette In-flight automated externaldefibrillatoruseandconsultationpatterns.PrehospEmerg Care2010;14:235–9.
- Bertrand C, Rodriguez Redington P, Lecarpentier E, et al. Preliminary report on AED deployment on theentire Air Francecommercial fleet: a joint venturewith Paris XIIUniversity Resuscitation2004;63: 175–81.
- HunterA.Willyouvolunteerin-flightmedicalcare?CanMedAssocJ 1980;123:137–40.
- Emergencymedicalequipmenttraining,advisorycircularno. 121-34B; Availablefrom: http://www.faa.gov/documentLibrary/media/Advisory Circular/AC121-34B.pdf.
- Hinkelbein J, Neuhaus C, Wetsch WA, et al. Emergencymedicalequipment on board German JTravelMed2014;21:318–23.
- Emergency medical equipment, advisory circular 121-33B; 2006. Available from: http://www.faa.gov/documentLibrary/media/Advisory Circular/AC12133B.pdf.
- Commission Regulation(EC) No 859/2008 of 20 August 2008 amending Council Regulation (EEC) No 3922/91 as regardscommon technicalrequirementsand administrative procedures applicable to commercial transportation by aeroplane. Off J Eur Union 2008. Available from: http://eur-lex.europa.eu/legalcontent/EN/ALL/?uri=CELEX:32008R0859.
- SandM,GambichlerT,SandD,ThrandorfC,AltmeyerP,BecharaFG.Emergency medicalkits onboardcommercialaircraft:acomparativestudy.TravelMed InfectDis2010;8:388–94.
- Skogvoll E, Bjelland E, Thorarinsson B. Helicopter emergency medical service in out-of- hospital cardiac arrest – a 10-year population-based Acta Anaesthesiol Scand 2000;44:972–9.
- Lyon RM, Nelson Helicopter emergency medical services (HEMS) response to out-of- hospitalcardiacarrest.ScandJTraumaResuscEmergMed2013;21:1.
- RittenbergerJC,HostlerDP,TobinT,GainesJ,CallawayCW.PredictorsofROSC inwitnessed Resuscitation2008;76:43–6.
- Pietsch U, Lischke V, Pietsch Benefitofmechanicalchestcompressiondevices inmountain HEMS:lessonslearnedfrom1yearofexperienceandevaluation. AirMedJ2014;33:299–301.
- OmoriK,SatoS,SumiY,etal.TheanalysisofefficacyforAutoPulsesystemin Resuscitation2013;84:1045–50.
- Harmon KG, Asif IM, Klossner D, Drezner JA. Incidence of sudden cardiac death in National Circulation 2011;123:1594–600.
- ChandraN,PapadakisM,SharmaS.Preparticipationscreeningofyoungcompetitiveathletesfor PhysSportsmed2010;38:54–63.
- Angelini P. Coronary artery anomalies: an entity in search of an identity. Circulation 2007;115:1296–305.
- Maron BJ, Doerer JJ, Haas TS, Tierney DM, Mueller FO. Suddendeaths in young competitive athletes:analysisof 1866 deathsintheUnitedStates, 1980–2006. Circulation2009;119:1085–
- Maron BJ, Gohman TE, Kyle SB, Estes III NA, Link MS. Clinical profile and spectrum of JAMA2002;287:1142–6.
- MaronBJ,HaasTS,AhluwaliaA,GarberichRF,EstesIIINA,LinkMS.Increasing survivalrate HeartRhythm2013;10:219–23.
- Maron BJ, Friedman RA, Kligfield P, et al. Assessment of the 12-lead electrocardiogram as a screening test for detection of cardiovascular disease in healthy general populations of young people(12–25yearsofage):ascientific statementfromtheAmericanHeartAssociationandthe AmericanCollegeof Cardiology.JAmCollCardiol2014;64:1479–514.
- Lin CY, Wang YF, Lu TH, Kawach I. Unintentional drowning mortality, by age and body of water:ananalysisof60countries.InjPrev2015;21:e43–50.
- VenemaAM,GroothoffJW,BierensJJ.Theroleofbystandersduringrescueand resuscitationof Resuscitation2010;81:434–9.
- Szpilman D, Webber J, Quan L, et Creating a drowning chain of survival. Resuscitation 2014;85:1149–52.
- BierensJ.Drowning.Prevention,rescue,treatment.2nded.Heidelberg: Springer;2014. Global Report on Drowning. PreventingaLeadingKiller;2014.Availablefrom: http://www.who.int/violence./drowning.report/Finalreportfullweb.pdf.
- RaczE,KonczolF,MeszarosH,etal.Drowning-relatedfatalitiesduringa5-year period(2008–2012)inSouth-WestHungary–aretrospectivestudy.JForensic LegMed2015;31:7–11.
- Halik R,PoznanskaA,SerokaW,WojtyniakB.AccidentaldrowningsinPoland in2000–2012. PrzeglEpidemiol2014;68:493–9,591–4.
- Claesson A, Lindqvist J, Ortenwall P, Herlitz J. Characteristics of lifesavingfrom drowning as reportedbytheSwedishFireandRescueServices1996–2010. Resuscitation2012;83:1072–7.
- Idris AH, Berg RA, Bierens J, Recommendedguidelinesforuniformreportingofdatafrom drowning:the“Utsteinstyle”.Resuscitation2003;59:45–57.
- IdrisAH,BergRA,BierensJ,etal.Recommendedguidelinesforuniform reportingofdatafrom drowningthe“Utsteinstyle”.Circulation2003;108: 2565–74.
- LayonAJ,ModellJH.Drowning:update2009.Anesthesiology2009;110: 1390–401.
- SzpilmanD,BierensJJ,HandleyAJ,OrlowskiJP.Drowning.NEnglJMed 2012;366:2102–10.
- SzpilmanD,SoaresM.In-waterresuscitation–isitworthwhile?Resuscitation 2004;63:25–31.
- Quan L, Wentz KR, Gore EJ, Copass Outcome and predictors of outcome in pediatric submersion victims receiving prehospital care in King County, Washington. Pediatrics 1990;86:586–93.
- Mtaweh H, Kochanek PM, Carcillo JA, Bell MJ, Fink EL. Patterns of multiorgan dysfunction Resuscitation2015;90:91–6.
- KyriacouDN,ArcinueEL,PeekC,KrausJF.Effectofimmediateresuscitationon childrenwith Pediatrics1994;94:137–42.
- Szpilman Near-drowninganddrowningclassification: aproposaltostratify mortalitybased ontheanalysisof1,831cases.Chest1997;112:660–5.
- Wallis BA, Watt K, Franklin RC, Taylor M, Nixon JW, Kimble RM. Interventions associated with drowning prevention in children and adolescents: systematic literature review. Inj Prev 2015;21:195–204.
- Leavy JE, Crawford G, Portsmouth L, Recreationaldrowningpreventioninterventionsfor adults,1990–2012:areview.JCommunityHealth 2015;40:725–35.
- VahataloR,LunettaP,OlkkolaKT,SuominenPK.Drowninginchildren:Utstein stylereporting ActaAnaesthesiolScand2014;58:604–10.
- ClaessonA,LindqvistJ,HerlitzJ.Cardiacarrestduetodrowning–changesover timeandfactors Resuscitation2014;85:644–8.
- Dyson K, Morgans A, Bray J, Matthews B, Smith Drowningrelated out-of-hospitalcardiac arrests:characteristicsandoutcomes.Resuscitation 2013;84:1114–8.
- Bierens JJ, van der Velde EA, van Berkel M, van Zanten JJ. Submersion in The Netherlands: AnnEmerg Med1990;19:1390–5.’
- Franklin RC, Pearn Drowning for love: the aquatic victim-instead-of-rescuer syndrome: drowning fatalities involving those attempting to rescue a child. J Paediatr Child Health 2011;47:44–7.
- Perkins GD, Travers AH, Considine J, et Part 3: Adult basic life support and automated external defibrillation: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation 2015;95:e43–70.
- Tipton MJ, Golden FS. A proposed decision-making guide for the search, rescue and resuscitation of submersion (head under) victims based on expert opinion. Resuscitation 2011;82:819–24.
- Wanscher M, Agersnap L, Ravn J, et al. Outcome of accidental hypothermia with or without circulatory arrest: experience from the Danish Praesto Fjord boating accident. Resuscitation 2012;83:1078–84.
- KieboomJK,VerkadeHJ,BurgerhofJG,etal.Outcomeafterresuscitationbeyond 30minutesin drownedchildrenwithcardiacarrestandhypothermia: Dutch nationwideretrospectivecohort BMJ2015;350:h418.
- Perkins In-water resuscitation: a pilot evaluation. Resuscitation 2005;65:321–4.
- Winkler BE, Eff AM, Ehrmann U, et al. Effectiveness and safety of in-water resuscitation performed by lifeguards and laypersons: a crossover manikin study. Prehosp Emerg Care 2013;17:409–15.
- Watson RS, Cummings P, Quan L, Bratton S, Weiss NS. Cervical spine injuries among JTrauma2001;51:658–62.
- March NF, Matthews RC. Feasibility study of CPR in the water. Undersea Biomed Res 1980;7:141–8.
- March NF, Matthews RC. New techniques in external cardiac compressions. Aquatic JAMA1980;244:1229–32.
- Barcala-Furelos R, Abelairas-Gomez C, Romo-Perez V, Palacios-Aguilar Effect ofphysical fatigueonthequalityCPR:awaterrescuestudyoflifeguards:physicalfatigueandqualityCPRin awaterrescue.AmJEmergMed2013;31:473–7.
- Claesson A, Karlsson T, Thoren AB, Herlitz Delay and performance of cardiopulmonary resuscitationinsurflifeguardsaftersimulatedcardiacarrest duetodrowning. Am JEmergMed 2011;29:1044–50.
- Manolios N, Mackie I. Drowning and near-drowning on Australian beaches patrolled by life- savers:a10-yearstudy,1973–1983.MedJAust 1988;148:165–7,170–1.
- Baker PA, Webber JB. Failure to ventilate with supraglottic airways after drowning. Anaesth IntensiveCare2011;39:675–7.
- Montenij LJ, de Vries W, Schwarte L, Bierens Feasibility of pulse oximetry in the initial prehospital management of victims of drowning: a preliminary study. Resuscitation 2011;82:1235–8.
- Moran I, Zavala E, Fernandez R, Blanch L, Mancebo Recruitmentmanoeuvres in acutelung injury/acuterespiratorydistresssyndrome.EurRespirJSuppl 2003;42:37s–42s.
- Wyatt JP, Tomlinson GS, Busuttil A. Resuscitation of drowningvictims in southeast Scotland. Resuscitation1999;41:101–4.
- BolteRG,BlackPG,BowersRS,ThorneJK,CorneliHM.Theuseofextracorporeal rewarming JAMA1988;260:377–9.
- SchmidtU,FritzKW,KasperczykW,TscherneH.Successfulresuscitationofa childwithsevere Prehospital DisasterMed1995;10:60–2.
- OehmichenM,HennigR,MeissnerC.Near-drowningandclinicallaboratory changes.LegMed (Tokyo)2008;10:1–5.
- Serumelectrolytechangesinnear-drowningvictims.JAMA 1985;253:557.
- GregorakosL,MarkouN,PsalidaV,etal.Near-drowning:clinicalcourseoflung injuryinadults. Lung2009;187:93–7.
- The Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as comparedwithtraditionaltidalvolumesforacutelung injury and theacuterespiratorydistress NEnglJMed2000;342: 1301–8.
- SutherasanY,PenuelasO,MurielA,etal.Managementandoutcomeofmechanicallyventilated CritCare2015;19:215.
- Eich C, Brauer A, Timmermann A, et Outcome of 12 drowned children with attempted resuscitationoncardiopulmonarybypass:ananalysisofvariables basedonthe“UtsteinStylefor Drowning”.Resuscitation2007;75:42–52.
- Guenther U, Varelmann D, Putensen C, Wrigge H. Extended therapeutic hypothermia for several days duringextracorporealmembrane-oxygenation afterdrowning and Twocasesofsurvivalwithnoneurological sequelae.Resuscitation2009;80:379–81.
- Kim KI, Lee WY, Kim HS, Jeong JH, Ko HH. Extracorporeal membrane oxygenation in near- drowning patients with cardiac or pulmonary failure. Scand J Trauma Resusc Emerg Med 2014;22:77.
- Champigneulle B, Bellenfant-Zegdi F, Follin A, et al. Extracorporeal life support (ECLS) for refractorycardiacarrestafterdrowning:an11-yearexperience. Resuscitation2015;88:126–31.
- Wood Towardsevidencebasedemergencymedicine: best BETsfromthe ManchesterRoyal InfirmaryBET.1,prophylacticantibioticsinnear-drowning. EmergMedJ2010;27:393–4.
- Van Berkel M, Bierens JJLM, Lie RLK, et Pulmonaryoedema, pneumonia and mortality in submersionvictimsaretrospectivestudyin125patients.Intensive CareMed1996;22:101–7.
- Davies KJ, Walters JH, Kerslake IM, Greenwood R, Thomas Early antibiotics improve survivalfollowingout-ofhospitalcardiacarrest.Resuscitation 2013;84:616–9.
- Tadie JM, Heming N, Serve E, et Drowning associated pneumonia: a descriptive cohort. Resuscitation2012;83:399–401.
- Proceedings of the 2005 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Resuscitation 2005;67:157–341.
- Paal P, Ellerton J, Sumann G, et al. Basic life support ventilation in mountain rescue. Official recommendationsoftheInternationalCommissionforMountain EmergencyMedicine(ICAR MEDCOM).HighAltMedBiol2007;8:147–54.
- ElsensohnF,SoterasI,ResitenO,EllertonJ,BruggerH,PaalP.Equipmentof medicalbackpacks HighAltMedBiol2011;12:343–7.
- Elsensohn F, Agazzi G, Syme D, et al. The use of automated external defibrillators and public access defibrillators in the mountains: official guidelines of the international commission for mountainemergencymedicineICAR-MEDCOM. WildernessEnvironMed2006;17:64–6.
- BruggerH,ElsensohnF,SymeD,SumannG,FalkM.Asurveyofemergency medicalservicesin mountain areas of Europe and North America: official recommendations of the International Commission for Mountain Emergency Medicine (ICAR Medcom). High Alt Med Biol 2005;6:226–37.
- Tomazin I, Ellerton J, Reisten O, Soteras I, Avbelj M, International Commission for Mountain Emergency Medicine. Medical standards for mountain rescue operations using helicopters: officialconsensusrecommendationsoftheInternationalCommissionforMountainEmergency Medicine(ICARMEDCOM). HighAltMedBiol2011;12:335–41.
- Pietsch U, Lischke V, Pietsch C, Kopp KH. Mechanical chest compressions in an avalanche victim with cardiac arrest: an option for extreme mountain rescue Wilderness EnvironMed2014;25:190–3.
- Ellerton J, Gilbert H. Should helicopters have a hoist or ‘long-line’ capability to perform mountainrescueintheUK?EmergMedJ2012;29:56–9.
- Klemenc-KetisZ,TomazinI,KersnikJ.HEMSinSlovenia:onecountry,four models,different AirMedJ2012;31:298–304.
- Tomazin I, Vegnuti M, Ellerton J, Reisten O, Sumann G, Kersnik J. Factors impacting on the ScandJTraumaResuscEmergMed 2012;20:56.
- Wang JC, Tsai SH, Chen YL, et The physiologicaleffects and quality of chest compressions duringCPRatsealevelandhighaltitude.AmJEmergMed 2014;32:1183–8.
- Suto T, Saito S. Considerations for resuscitation at high altitude in elderly and untrained AmJEmergMed2014;32:270–6.
- NaraharaH,KimuraM,SutoT,etal.Effectsofcardiopulmonaryresuscitation athighaltitudeson the physical condition of untrained and unacclimatized Wilderness Environ Med 2012;23:161–4.
- Boyd J, Brugger H, Shuster M. Prognostic factors in avalanche resuscitation: a systematic Resuscitation2010;81:645–52.
- Locher T, Walpoth BH. Differential diagnosis of circulatory failure in hypothermic avalanche victims: retrospective analysis of 32 avalanche accidents. Praxis (Bern 1994) 1996;85:1275–
- Grissom CK, Radwin MI, Scholand MB, Harmston CH, Muetterties MC, Bywater Hypercapnia increases core temperature cooling rate during snow burial. J Appl Physiol 2004;96:1365–70.
- Oberhammer R, Beikircher W, Hormann C, et al. Full recovery of an avalanche victim with profound hypothermia and prolonged cardiac arrest treated by extracorporeal re-warming. Resuscitation2008;76:474–80.
- Mair P, Brugger H, Mair B, Moroder L, Ruttmann Is extracorporealrewarming indicated in avalanchevictimswithunwitnessedhypothermiccardiorespiratoryarrest? High Alt Med Biol 2014;15:500–3.
- Boue Y, Payen JF, Brun J, et al. Survivalafteravalanche-inducedcardiacarrest. Resuscitation 2014;85:1192–6.
- Hilmo J,Naesheim T,Gilbert M.Nobodyisdeaduntilwarmanddead:prolongedresuscitationis warranted in arrested hypothermic victims also in remote areas – a retrospective study from Resuscitation 2014;85:1204–11.
- Brugger H, Sumann G, Meister R, et Hypoxia and hypercapnia during respiration into an artificialairpocketinsnow:implicationsforavalanchesurvival. Resuscitation2003;58:81–8.
- Haegeli P, Falk M, Brugger H, Etter HJ, Boyd Comparison of avalanche survival patterns in CanadaandSwitzerland.CanMedAssocJ2011;183: 789–95.
- Boyd J, Haegeli P, Abu-Laban RB, Shuster M, Butt Patterns of death among avalanche fatalities:a21-yearreview.CanMedAssocJ2009;180:507–12.
- Brugger H, Durrer B, Elsensohn F, et al. Resuscitation of avalanche victims: evidence-based guidelines of the international commission for mountain emergency medicine (ICAR MEDCOM): intendedforphysiciansandother Resuscitation 2013;84:539–46.
- BruggerH,PaalP,BoydJ.Prehospitalresuscitationoftheburiedavalanche victim.HighAltMed Biol2011;12:199–205.
- Kottmann A, Blancher M, Spichiger T, et The Avalanche Victim Resuscitation Checklist, a newconceptforthemanagementofavalanchevictims.Resuscitation2015;91:e7–8.
- Budnick LD. Bathtub-related electrocutions in the United States, 1979 to 1982. JAMA 1984;252:918–20.
- Lightning-associated deaths – United States, 1980–1995. MMWR Morb Mortal Wkly Rep 1998;47:391–4.
- GeddesLA,BourlandJD,FordG.ThemechanismunderlyingsuddendeathfromMedInstrum1986;20:303–15.
- ZafrenK,DurrerB,HerryJP,BruggerH.Lightninginjuries:preventionand on-sitetreatmentin Officialguidelinesofthe InternationalCommissionforMountain Emergency Medicine and the Medical Commission of the International Mountaineering and ClimbingFederation (ICARandUIAAMEDCOM).Resuscitation2005;65:369–72.
- CheringtonM,Lightninginjuries.AnnEmergMed1995;25:517–9.
- Fahmy FS, Brinsden MD, Smith J, Frame Lightning: the multisystem group injuries. J Trauma1999;46:937–40.
- PattenBM.Lightningandelectricalinjuries.NeurolClin1992;10:1047–58.
- Browne BJ, Gaasch Electrical injuries and lightning. Emerg Med Clin North Am 1992;10:211–29.
- Kleiner JP, Wilkin Cardiac effects of lightning stroke. JAMA 1978;240:2757–9.
- Lichtenberg R, Dries D, Ward K, Marshall W, Scanlon P. Cardiovascular effects of lightning JAmCollCardiol1993;21:531–6.
- CooperMA. Emergentcareoflightningandelectricalinjuries.SeminNeurol 1995;15:268–78.
- Milzman DP, Moskowitz L, Hardel M. Lightning strikes at a mass gathering. South Med J 1999;92:708–10. 1995;15:323–8.
- CooperMA.Lightninginjuries:prognosticsignsfordeath.AnnEmergMed 1980;9:134–8.
- Kleinschmidt-DeMasters BK. Neuropathology of lightning-strike injuries. Semin Neurol 1995;15:323–8.
- Cherington M, McDonough G, Olson S, Russon R, Yarnell PR. Lichtenberg figures and lightning: case reports and review of the literature. Cutis 2007; 80:141–3.
- EpperlyTD,StewartJR.Thephysicaleffectsoflightninginjury.JFamPract 1989;29:267–72.
- Duclos PJ, Sanderson LM. An epidemiological description of lightning-related deaths in the United States. IntJEpidemiol1990;19:673–9. 2003;49:297–8.
- WhitcombD,MartinezJA,DaberkowD.Lightninginjuries.SouthMedJ 2002;95:1331–4.
- Goldman RD, Einarson A, Koren G. Electric shock during pregnancy. Can Fam Physician
- BlumenthalR,SaaymanG.Bonemarrowembolismtothelunginelectrocution: AmJForensicMedPathol2014;35:170–1.
- El Sayed M, Tamim H, Mann NC. Description of procedures performed on patients by emergency medical services during mass casualty incidents in the United States. Am J Emerg Med2015;33:1030–6.
- World Disasters Report 2014; Available from: https://www.ifrc.org/ world-disasters- report-2014/data.
- SchenkE,WijetungeG,MannNC,LernerEB,LongthorneA,DawsonD.Epidemiologyofmass PrehospEmergCare 2014;18:408–16.
- Tokuda Y, Kikuchi M, Takahashi O, Stein Prehospital management of sarin nerve gas terrorism in urban settings: 10 years of progress after the Tokyo subway sarin attack. Resuscitation2006;68:193–202.
- LamhautL,DagronC,AprioteseiR,etal.Comparisonofintravenousand intraosseousaccessby pre-hospital medical emergency personnel with and without CBRN protective equipment. Resuscitation2010;81:65–8.
- Castle N, Pillay Y, Spencer N. Comparisonofsixdifferentintubationaidsforuse whilewearing CBRN-PPE:amanikinstudy.Resuscitation2011;82:1548–52.
- Castle N, Bowen J, Spencer N. Does wearing CBRN-PPE adversely affect the ability for clinicianstoaccurately,safely, andspeedilydrawupdrugs?Clin Toxicol(Phila)2010;48:522–
- CrossKP,PetryMJ,CiceroMX. AbetterSTARTforlow-acuityvictims:datadrivenrefinement PrehospEmergCare2015;19: 272–8.
- SALT mass casualty triage: concept endorsed by the American College of Emergency Physicians,AmericanCollegeof SurgeonsCommitteeon Trauma,AmericanTraumaSociety, National Association of EMS Physicians, National Disaster Life Support Education Consortium, and State and Territorial Injury Prevention Directors Association. Disaster Med PublicHealthPrep 2008;2:245–6.
- ConeDC,SerraJ,BurnsK,MacMillanDS,KurlandL,VanGelderC.Pilottestof theSALTmass PrehospEmergCare2009;13:536–40.
- RisaviBL,TerrellMA,LeeW,HolstenJrDL.Prehospitalmass-casualtytriage training-written versus moulage scenarios: how much do EMS providers retain? Prehosp Disaster Med 2013;28:251–6.
- Knight JF, Carley S, Tregunna B, et Serious gaming technology in major incident triage training:apragmaticcontrolledtrial.Resuscitation 2010;81:1175–9.
- Postma IL, Weel H, Heetveld MJ, et al. Mass casualty triage after an airplane crash near Injury2013;44:1061–7.
- JonesN,WhiteML,TofilN,etal.Randomizedtrialcomparingtwomasscasualty triagesystems (JumpSTARTversusSALT) inapediatricsimulatedmasscasualty PrehospEmergCare 2014;18:417–23.
- Masoli M, Fabian D, Holt S, Beasley Theglobalburdenofasthma: executivesummaryofthe GINADisseminationCommitteereport.Allergy 2004;59:469–78.
- To T, Stanojevic S, Moores G, Globalasthmaprevalenceinadults:findings fromthecross- sectionalworldhealthsurvey.BMCPublicHealth2012;12:204.
- Pearce N, Ait-Khaled N, Beasley R, et al. Worldwide trends in the prevalence of asthma symptoms:phaseIIIoftheInternationalStudyofAsthmaandAllergies inChildhood(ISAAC). Thorax2007;62:758–66.
- Anandan C, Nurmatov U, van Schayck OC, Sheikh A. Is the prevalence of asthma declining?
- Systematicreviewofepidemiologicalstudies.Allergy 2010;65:152–67.
- CohenS,BerkmanN,AvitalA,etal.Declineinasthmaprevalenceandseverity inIsraelovera10- Respiration2015;89:27–32.
- Mikalsen IB, Skeiseid L, Tveit LM, Engelsvold DH, Oymar K. Decline in admissions for childhood asthma, a 26-year period population-based study. Pediatr Allergy Immunol 2015, http://dx.doi.org/10.1111/pai.12372.Mar18.[Epub aheadofprint].
- Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, EurRespirJ 2014;43:343–73.
- RomagnoliM,CaramoriG,BraccioniF,etal.Near-fatalasthmaphenotypein theENFUMOSA ClinExpAllergy2007;37:552–7.
- Alvarez GG, Schulzer M, Jung D, Fitzgerald A systematicreview of risk factorsassociated withnear-fatalandfatalasthma.CanRespirJ2005;12:265–70.
- Turner MO, Noertjojo K, Vedal S, Bai T, Crump S, Fitzgerald JM. Risk factors for near-fatal asthma: a case–control study in hospitalized patients with asthma. Am J Respir Crit Care Med 1998;157:1804–9.
- Ernst P, Spitzer WO, Suissa S, et al. Risk of fatal and near-fatal asthma in relation to inhaled JAMA1992;268:3462–4.
- SuissaS,BlaisL,ErnstP.Patternsofincreasingbeta-agonistuseandtheriskof fatalornear-fatal EurRespirJ1994;7:1602–9.
- Alvarez GG, Fitzgerald JM. A systematic review of the psychological risk factors associated Respiration2007;74:228–36.
- Sturdy PM, Victor CR, Anderson HR, et Psychological, social and health behaviour risk factorsfordeathscertifiedasasthma:anationalcase–control study.Thorax2002;57:1034–9.
- Roberts G, Patel N, Levi-Schaffer F, Habibi P, Lack G. Food allergy as a risk factor for life- threatening asthma in childhood: a case–controlled J Allergy Clin Immunol 2003;112:168–74.
- Why asthma still kills: the national review of asthma deaths (NRAD). Confidential Enquiry Report 2014; 2014. Available from: http://www.rcplondon.ac. uk/sites/default/files/why- asthma-still-kills-full-report.pdf.
- TsaiCL,LeeWY,HananiaNA,CamargoJrCA.Age-relateddifferencesinclinicaloutcomesfor acuteasthmaintheUnitedStates,2006–2008.JAllergyClin Immunol2012;129,1252–8.e1.
- Williams TJ, Tuxen DV, Scheinkestel CD, Czarny D, Bowes G. Risk factors for morbidity in Am RevRespirDis1992;146:607– 15.
- KokturkN,DemirN,KervanF,DincE,KoybasiogluA,TurktasH.Asubglotticmassmimicking near-fatalasthma:achallengeofdiagnosis.JEmergMed 2004;26:57–60.
- Globalstrategyforasthmamanagementandprevention2009;2009[accessed 06.10].
- SIGN 141 British guideline on the management of asthma; Available from: http://www.sign.ac.uk/pdf/SIGN141.pdf.
- Rodrigo GJ, Nannini LJ. Comparisonbetweennebulizedadrenaline and beta2 agonistsforthe treatment of acute asthma. A meta-analysis of randomized Am J Emerg Med 2006;24:217–22.
- Rodrigo G, Rodrigo C, Burschtin O. A meta-analysis of the effects of ipratropium bromide in AmJMed1999;107:363–70.
- Theuseofipratropiumbromideforthemanagementofacute asthmaexacerbationin adultsandchildren:asystematicreview.JAsthma 2001;38:521–30.
- Mohammed S, Goodacre S. Intravenous and nebulised magnesium sulphate for acute asthma: systematicreviewandmeta-analysis.EmergMedJ 2007;24:823–30.
- Powell C, Dwan K, Milan SJ, et Inhaledmagnesiumsulfate in thetreatment of acuteasthma. CochraneDatabaseSystRev2012;12:CD003898.
- BradshawTA,MatusiewiczSP,CromptonGK,InnesJA,GreeningAP.Intravenousmagnesium sulphate provides no additive benefit to standard management in acute asthma. Respir Med 2008;102:143–9.
- Goodacre S, Cohen J, Bradburn M, et al. Intravenous or nebulised magnesium sulphate versus standard therapy for severe acute asthma (3Mg trial): a doubleblind, randomised controlled trial. LancetRespirMed2013;1:293–300.
- Kew KM, Kirtchuk L, Michell CI. Intravenous magnesium sulfate for treating adults with acute asthmaintheemergencydepartment.CochraneDatabase SystRev2014;5:CD010909.
- RoweBH,SpoonerCH,DucharmeFM,BretzlaffJA,BotaGW.Corticosteroids forpreventing relapse following acute exacerbations of asthma. Cochrane Database Syst Rev 2001:CD000195.
- Ratto D, Alfaro C, Sipsey J, Glovsky MM, Sharma OP . Are intravenous corticosteroids required instatusasthmaticus?JAMA1988;260:527–9.
- TraversA,JonesAP,KellyK,BarkerSJ,CamargoCA,RoweBH.Intravenous beta2-agonists foracuteasthmaintheemergencydepartment.Cochrane DatabaseSystRev2001:CD002988.
- Cowman S, Butler J. Towards evidence based emergency medicine: best BETs from the Manchester Royal Infirmary. BET 3. The use of intravenous aminophylline in addition to beta-agonistsandsteroidsinacuteasthma.EmergMed J2008;25:289–90.
- Parameswaran K, Belda J, Rowe BH. Addition of intravenous aminophylline to beta2-agonists inadultswithacuteasthma.CochraneDatabaseSystRev 2000:CD002742.
- Kuitert LM, Watson D. Antileukotrienes as adjunctive therapy in acute asthma. Drugs 2007;67:1665–70.
- Camargo Jr CA, Gurner DM, Smithline HA, et al. A randomized placebocontrolled study of intravenous montelukast for the treatment of acute asthma. J Allergy Clin Immunol 2010;125:374–80.
- Watts K, Chavasse RJ. Leukotriene receptor antagonists in addition to usual care for acute asthmainadultsandchildren.CochraneDatabaseSystRev 2012;5:CD006100.
- RodrigoGJ,RodrigoC,PollackCV,RoweB.Useofhelium–oxygenmixtures inthetreatmentof acuteasthma:asystematicreview.Chest2003;123: 891–6.
- GuptaD,KeoghB,ChungKF,etal.Characteristicsandoutcomeforadmissions toadult,general critical care units with acute severe asthma: a secondary analysis of the ICNARC Case Mix ProgrammeDatabase.CritCare2004;8: R112–21.
- BrennerB,CorbridgeT,KazziA.Intubationandmechanicalventilation oftheasthmaticpatient inrespiratoryfailure.JAllergyClinImmunol 2009;124:S19–28.
- Antonelli M, Pennisi MA, Montini L. Clinical review: noninvasive ventilation in the clinicalsetting–experiencefromthepast10years.CritCare 2005;9:98–103.
- LimWJ,MohammedAkramR,CarsonKV,etal.Non-invasivepositivepressure ventilationfor treatment of respiratory failure due to severe acute exacerbations of asthma. Cochrane Database SystRev2012;12:CD004360.
- LeathermanJW,McArthurC,ShapiroRS.Effectofprolongationofexpiratorytimeondynamic hyperinflation in mechanically ventilated patients with severe asthma. Crit Care Med 2004;32:1542–5.
- Bowman FP, Menegazzi JJ, Check BD, Duckett TM. Lower esophageal sphincter pressure duringprolongedcardiacarrestandresuscitation.AnnEmergMed 1995;26:216–9.
- Lapinsky SE, Leung RS. Auto-PEEP and electromechanical dissociation. N Engl J Med 1996;335:674.
- Rogers PL, Schlichtig R, Miro A, Pinsky M. Auto-PEEP during CPR. An “occult” cause of electromechanicaldissociation?Chest1991;99:492–3.
- RosengartenPL,TuxenDV,DziukasL,ScheinkestelC,MerrettK,BowesG.Circulatoryarrest induced by intermittent positive pressure ventilation in a patient with severe asthma. Anaesth IntensiveCare1991;19:118–21.
- Sprung J, Hunter K, Barnas GM, Bourke DL. Abdominal distention is not always a sign of esophagealintubation:cardiacarrestdueto“auto-PEEP”.AnesthAnalg 1994;78:801–4.
- Harrison R. Chest compression first aid for respiratory arrest due to acute asphyxic asthma. EmergMedJ2010;27:59–61.
- DeakinCD,McLarenRM,PetleyGW,ClewlowF,Dalrymple-HayMJ.Effectsof positiveend-expiratory pressure on transthoracic impedance – implications for defibrillation. Resuscitation 1998;37:9–12.
- Galbois A, Ait-Oufella H, Baudel JL, et al. Pleural ultrasound compared to chest radiographic detectionofpneumothoraxresolutionafterdrainage.Chest 2010;138:648–55.
- MabuchiN,TakasuH,ItoS,etal.Successfulextracorporeallungassist(ECLA) forapatientwith severeasthmaandcardiacarrest.ClinIntensiveCare 1991;2:292–4.
- Martin GB, Rivers EP, Paradis NA, Goetting MG, Morris DC, Nowak RM. Emergency department cardiopulmonary bypass in the treatment of human cardiac arrest. Chest 1998;113:743–51.
- Mabvuure NT, Rodrigues JN. External cardiac compression during cardiopulmonary resuscitation of patients with left ventricular assist devices. Interact Cardiovasc Thorac Surg 2014;19:286–9.
- HubnerP,MeronG,KurkciyanI,etal.Neurologiccausesofcardiacarrestand outcomes.JEmerg Med2014;47:660–7.
- Thurman DJ, Hesdorffer DC, French JA. Sudden unexpected death in epilepsy: assessing the publichealthburden.Epilepsia2014;55:1479–85.
- Arnaout M, Mongardon N, Deye N, et al. Out-of-hospital cardiac arrest from brain cause: epidemiology, clinical features, and outcome in a multicenter cohort. Crit Care Med 2015;43:453–60.
- Skrifvars MB, Parr MJ. Incidence, predisposing factors, management and survival following cardiac arrest due to subarachnoid haemorrhage: a review of the literature. Scand J Trauma ResuscEmergMed2012;20:75.
- MitsumaW,ItoM,KodamaM,etal.Clinicalandcardiacfeaturesofpatients withsubarachnoid haemorrhagepresentingwithout-of-hospitalcardiac arrest.Resuscitation2011;82:1294–7.
- Sandroni C, Dell’Anna AM. Out-of-hospital cardiac arrest from neurologic cause: recognition andoutcome.CritCareMed2015;43:508–9.
- Noritomi DT, de Cleva R, Beer I, et al. Doctors awareness of spontaneous subarachnoid haemorrhageasacauseofcardiopulmonaryarrest.Resuscitation 2006;71:123–4.
- Sandroni C, Adrie C, Cavallaro F, et al. Are patients brain-dead after successful resuscitation from cardiac arrest suitable as organ donors? A systematic review. Resuscitation 2010;81:1609–14.
- Jain R, Nallamothu BK, Chan PS. American Heart Association National Registry of Cardiopulmonary Resuscitation: i. Body mass index and survival after inhospital cardiac arrest. CircCardiovascQualOutcomes2010;3:490–7.
- Testori C, Sterz F, Losert H, et al. Cardiac arrest survivors with moderate elevated body mass indexmayhaveabetterneurologicaloutcome:acohortstudy. Resuscitation2011;82:869–73.
- Obesity and overweight. Fact sheet no. 311; 2015. Available from: http://www.who.int/mediacentre/factsheets/fs311/en/.
- Adams KF, Schatzkin A, Harris TB, et al. Overweight, obesity, and mortality in a large prospectivecohortofpersons50to71yearsold.NEnglJMed 2006;355:763–78.
- Romero-Corral A, Montori VM, Somers VK, et al. Association of bodyweight with total mortality and with cardiovascular events in coronary artery disease: a systematic review of cohortstudies.Lancet2006;368:666–78.
- Adabag S, Huxley RR, Lopez FL, et al. Obesity related risk of sudden cardiac death in the atherosclerosisriskincommunitiesstudy.Heart2015;101:215–21.
- DuflouJ,VirmaniR,RabinI,BurkeA,FarbA,SmialekJ.Suddendeathasaresult ofheartdisease inmorbidobesity.AmHeartJ1995;130:306–13.
- Nishisaki A, Maltese MR, Niles DE, et al. Backboards are important when chest compressions areprovidedonasoftmattress.Resuscitation2012;83: 1013–20.
- Bunch TJ, White RD, Lopez-Jimenez F, Thomas RJ. Association of body weight with total mortality and with ICD shocks among survivors of ventricular fibrillation in out-of-hospital cardiacarrest.Resuscitation2008;77:351–5.
- WhiteRD,BlackwellTH,RussellJK,JorgensonDB.Bodyweightdoesnotaffect defibrillation, resuscitation, or survival in patients with out-of-hospital cardiac arrest treated with a nonescalatingbiphasicwaveformdefibrillator.CritCare Med2004;32:S387–92.
- Sugerman H, Windsor A, Bessos M, Wolfe L. Intra-abdominal pressure, sagittal abdominal diameterandobesitycomorbidity.JInternMed1997;241:71–9.
- Holmberg TJ, Bowman SM, Warner KJ, et al. The association between obesity and difficult prehospitaltrachealintubation.AnesthAnalg2011;112:1132–8.
- Reminiac F, Jouan Y, Cazals X, Bodin JF, Dequin PF, Guillon A. Risks associated with obese patienthandlinginemergencyprehospitalcare.PrehospEmerg Care2014;18:555–7.
- KruskaP,KappusS,KernerT.Obesityinprehospitalemergencycare.AnasthesiolIntensivmed NotfallmedSchmerzther2012;47:556–62.
- Chalkias A, Xanthos T. The obesity paradox in cardiac arrest patients. Int J Cardiol 2014;171:101–2.
- TrendsinMaternalMortality:1990to2013.EstimatesbyWHO,UNICEF, UNFPA,TheWorld Bank and the United Nations Population Division; 2013. Available from: http://www.who.int/reproductivehealth/publications/ monitoring/maternal-mortality- 2013/en/.
- Lipman S, Cohen S, Einav S, et al. The Society for Obstetric Anesthesia and Perinatology consensus statement on the management of cardiac arrest in pregnancy. Anesth Analg 2014;118:1003–16.
- SoarJ,CallawayCW,AibikiM,etal.Part4:Advancedlifesupport:2015 internationalconsensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations.Resuscitation 2015.
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. LancetGlobHealth2014;2:e323–33.
- UK and Ireland confidential enquiries into maternal deaths and morbidity 2009–2012. Saving lives,improvingmothers’care;2014.
- Page-Rodriguez A, Gonzalez-Sanchez JA. Perimortem cesarean section of twin pregnancy: casereportandreviewoftheliterature.AcadEmergMed 1999;6:1072–4.
- Cardosi RJ, Porter KB. Cesarean delivery of twins during maternal cardiopulmonary arrest. ObstetGynecol1998;92:695–7.
- Mendonca C, Griffiths J, Ateleanu B, Collis RE. Hypotension following combined spinal- epidural anaesthesia for Caesarean section. Left lateral position vs. tilted supine position. Anaesthesia2003;58:428–31.
- Rees SG, Thurlow JA, Gardner IC, Scrutton MJ, Kinsella SM. Maternal cardiovascular consequences of positioning after spinal anaesthesia for Caesarean section: left 15 degree table tiltvs.leftlateral.Anaesthesia2002;57:15–20.
- BamberJH,DresnerM.Aortocavalcompressioninpregnancy:theeffectof changingthedegree and direction of lateral tilt on maternal cardiac output. Anesth Analg 2003;97:256–8, table of contents.
- Carbonne B, Benachi A, Leveque ML, Cabrol D, Papiernik E. Maternal position during labor: effects on fetal oxygen saturation measured by pulse oximetry. Obstet Gynecol 1996;88:797– 800.
- TamasP,SzilagyiA,JegesS,etal.Effectsofmaternalcentralhemodynamicson fetalheartrate patterns.ActaObstetGynecolScand2007;86:711–4.
- Abitbol MM. Supine position in labor and associated fetal heart rate changes. Obstet Gynecol 1985;65:481–6.
- KinsellaSM.Lateraltiltforpregnantwomen:why15degrees?Anaesthesia 2003;58:835–6.
- Goodwin AP, Pearce AJ. The human wedge. A manoeuvre to relieve aortocaval compression duringresuscitationinlatepregnancy.Anaesthesia 1992;47:433–4.
- Rees GA, Willis BA. Resuscitation in late pregnancy. Anaesthesia 1988;43:347–9.
- JonesSJ,KinsellaSM,DonaldFA.Comparisonofmeasuredandestimatedangles oftabletiltat Caesareansection.BrJAnaesth2003;90:86–7.
- Nanson J, Elcock D, Williams M, Deakin CD. Do physiological changes in pregnancy change defibrillationenergyrequirements?BrJAnaesth2001;87:237–9.
- ChiloiroM,DarconzaG, Piccioli E, De Carne M, Clemente C, Riezzo G. Gastric emptying and orocecal transit time in pregnancy.JGastroenterol 2001;36:538–43.
- O’Sullivan G. Gastric emptying during pregnancy and the puerperium. Int J Obstet Anesth 1993;2:216–24.
- Johnson MD, Luppi CJ, Over DC. Cardiopulmonary resuscitation. In: Gambling DR, Douglas MJ,editors.Obstetricanesthesiaanduncommondisorders. Philadelphia:W.B.Saunders;1998. p.51–74.
- IzciB,VennelleM,ListonWA,DundasKC,CalderAA,DouglasNJ.Sleepdisorderedbreathing andupperairwaysizeinpregnancyandpost-partum. EurRespirJ2006;27:321–7.
- Rahman K, Jenkins JG. Failed tracheal intubation in obstetrics: no more frequent but still managedbadly.Anaesthesia2005;60:168–71.
- Henderson JJ, Popat MT, Latto IP, Pearce AC. Difficult Airway Society guidelines for managementoftheunanticipateddifficultintubation.Anaesthesia 2004;59:675–94.
- Potts M, Prata N, Sahin-Hodoglugil NN. Maternal mortality: one death every 7 min. Lancet 2010;375:1762–3.
- Lewis G. The Confidential Enquiry into Maternal and Child Health (CEMACH). Saving mothers lives; reviewing maternal deaths to make motherhood safer 2003–05. The seventh report of the United Kingdom confidential enquiries into maternal deaths in the United Kingdom.London:CEMACH/RCOGPress;2007.
- American College of Obstetricians and Gynecologists. Optimizing protocols in obstetrics managementofobstetrichemorrhage;2012.
- WHO. WHO recommendations for the prevention and treatment of postpartum haemorrhage; 2012.
- GeogheganJ,DanielsJP,MoorePA,ThompsonPJ,KhanKS,GulmezogluAM. Cellsalvageat caesareansection:theneedforanevidence-basedapproach. BJOG2009;116:743–7.
- Bouwmeester FW, Bolte AC, van Geijn HP. Pharmacological and surgical therapy for primary postpartumhemorrhage.CurrPharmDes2005;11:759–73.
- Hofmeyr GJ, Abdel-Aleem H, Abdel-Aleem MA. Uterine massage for preventing postpartum haemorrhage.CochraneDatabaseSystRev2008:CD006431.
- Sekhavat L, Tabatabaii A, Dalili M, Farajkhoda T, Tafti AD. Efficacy of tranexamic acid in reducingbloodlossaftercesareansection.JMaternFetalNeonatalMed 2009;22:72–5.
- Phillips LE, McLintock C, Pollock W, et al. Recombinant activated factor VII in obstetric hemorrhage:experiencesfromtheAustralianandNewZealand HaemostasisRegistry.Anesth Analg2009;109:1908–15.
- BomkenC,MathaiS,BissT,LoughneyA,HanleyJ.RecombinantActivatedFactor VII(rFVIIa) in the management of major obstetric haemorrhage: a case series and a proposed guideline for use.ObstetGynecolInt2009;2009:364–843.
- DoumouchtsisSK,PapageorghiouAT,VernierC,ArulkumaranS.Management ofpostpartum hemorrhagebyuterineballoontamponade:prospectiveevaluationofeffectiveness.ActaObstet GynecolScand2008;87:849–55.
- Georgiou C. Balloon tamponade in the management of postpartum haemorrhage: a review. BJOG2009;116:748–57.
- El-Hamamy E, B-Lynch C. A worldwide review of the uses of the uterine compression suture techniques as alternative to hysterectomy in the management of severe post-partum haemorrhage.JObstetGynaecol2005;25:143–9.
- Hong TM, Tseng HS, Lee RC, Wang JH, Chang CY. Uterine artery embolization: an effective treatmentforintractableobstetrichaemorrhage.ClinRadiol 2004;59:96–101.
- Knight M. Peripartum hysterectomy in the UK: management and outcomes of the associated haemorrhage.BJOG2007;114:1380–7.
- Rossi AC, Lee RH, Chmait RH. Emergency postpartum hysterectomy for uncontrolled postpartumbleeding:asystematicreview.ObstetGynecol 2010;115:637–44.
- Y u S, Pennisi JA, Moukhtar M, Friedman EA. Placental abruption in association with advanced abdominalpregnancy.Acasereport.JReprodMed 1995;40:731–5.
- Ray P, Murphy GJ, Shutt LE. Recognition and management of maternal cardiac disease in pregnancy.BrJAnaesth2004;93:428–39.
- Abbas AE, Lester SJ, Connolly H. Pregnancy and the cardiovascular system. Int J Cardiol 2005;98:179–89.
- RoyalCollegeofObstetriciansandGynaecologists.Cardiacdiseaseinpregnancy;2011.
- James AH, Jamison MG, Biswas MS, Brancazio LR, Swamy GK, Myers ER. Acute myocardial infarction in pregnancy: a United States population-based study. Circulation 2006;113:1564–71.
- Ahearn GS, Hadjiliadis D, Govert JA, Tapson VF. Massive pulmonary embolism during pregnancysuccessfullytreatedwithrecombinanttissueplasminogen activator:acasereportand reviewoftreatmentoptions.ArchInternMed 2002;162:1221–7.
- Drenthen W, Pieper PG, Roos-Hesselink JW, et al. Outcome of pregnancy in women with congenitalheartdisease:aliteraturereview.JAmCollCardiol 2007;49:2303–11.
- SibaiB,DekkerG,KupfermincM.Pre-eclampsia.Lancet2005;365:785–99.
- Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol 2005;105:402–10.
- Duley L, Gulmezoglu AM, Henderson-Smart DJ. Magnesium sulphate and other anticonvulsantsforwomenwithpre eclampsia.CochraneDatabaseSystRev 2003:CD000025.
- Duley L, Henderson-Smart D. Magnesium sulphate versus phenytoin for eclampsia. Cochrane DatabaseSystRev2003:CD000128.
- Duley L, Henderson-Smart D. Magnesium sulphate versus diazepam for eclampsia. Cochrane DatabaseSystRev2003:CD000127.
- World Health Organization. WHO recommendations for Prevention and treatment of pre-eclampsiaandeclampsia;2011.
- Duley L, Henderson-Smart DJ, Walker GJ, Chou D. Magnesium sulphate versus diazepam for eclampsia.CochraneDatabaseSystRev2010:CD000127.
- Duley L, Henderson-Smart DJ, Chou D. Magnesium sulphate versus phenytoin for eclampsia. CochraneDatabaseSystRev2010:CD000128.
- Duley L, Gulmezoglu AM, Chou D. Magnesium sulphate versus lytic cocktail for eclampsia. CochraneDatabaseSystRev2010:CD002960.
- Duley L, Matar HE, Almerie MQ, Hall DR. Alternative magnesium sulphate regimens for womenwithpre-eclampsiaandeclampsia.CochraneDatabaseSyst Rev2010:CD007388.
- Knight M. Antenatal pulmonary embolism: risk factors, management and outcomes. BJOG 2008;115:453–61.
- Dapprich M, Boessenecker W. Fibrinolysis with alteplase in a pregnant woman with stroke. CerebrovascDis2002;13:290.
- Turrentine MA, Braems G, Ramirez MM. Use of thrombolytics for the treatment of thromboembolicdiseaseduringpregnancy.ObstetGynecolSurv 1995;50:534–41.
- Thabut G, Thabut D, Myers RP, et al. Thrombolytic therapy of pulmonary embolism: a meta-analysis.JAmCollCardiol2002;40:1660–7.
- PatelRK,FasanO,AryaR.Thrombolysisinpregnancy.ThrombHaemost 2003;90:1216–7.
- Conde-Agudelo A, Romero R. Amniotic fluid embolism: an evidence-based review. Am J ObstetGynecol2009;201,445e1–e4513.
- Fitzpatrick K, Tuffnell D, Kurinczuk J, Knight M. Incidence, risk factors, management and outcomes of amniotic-fluid embolism: a population-based cohort and nested case–control study.BJOG2015, http://dx.doi.org/10.1111/1471-0528.13300.Feb12.[Epubaheadofprint].
- Stanten RD, Iverson LI, Daugharty TM, Lovett SM, Terry C, Blumenstock E. Amniotic fluid embolism causing catastrophic pulmonary vasoconstriction: diagnosis by transesophageal echocardiogramandtreatmentbycardiopulmonarybypass.ObstetGynecol2003;102:496–8.
- Einav S, Kaufman N, Sela HY. Maternal cardiac arrest and perimortem caesarean delivery: evidenceorexpert-based?Resuscitation2012;83:1191–200.
- Dijkman A, Huisman CM, Smit M, et al. Cardiac arrest in pregnancy: increasing use of perimortemcaesareansectionduetoemergencyskillstraining?BJOG 2010;117:282–7.
- BaghirzadaL,BalkiM.Maternalcardiacarrestinatertiarycarecentreduring 1989–2011:acase series.CanJAnaesth2013;60:1077–84.
- Katz VL, Dotters DJ, Droegemueller W. Perimortem cesarean delivery. Obstet Gynecol 1986;68:571–6.
- American Heart Association in collaboration with International Liaison Committee on Resuscitation. Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency CardiovascularCare.Circulation2000;102: I1–384.
- Chapter 4; part 6: cardiac arrest associated with pregnancy.Cummins R, Hazinski M, Field J, editors.ACLS–thereferencetextbook.Dallas:AmericanHeart Association;2003.p.143–58.
- Katz V, Balderston K, DeFreest M. Perimortem cesarean delivery: were our assumptions correct?AmJObstetGynecol2005;192:1916–20,discussion 1920–1.
- Oates S, Williams GL, Rees GA. Cardiopulmonary resuscitation in late pregnancy. BMJ 1988;297:404–5.
- StrongTHJ,LoweRA.Perimortemcesareansection.AmJEmergMed 1989;7:489–94.
- BoydR,TeeceS.Towardsevidencebasedemergencymedicine:bestBETsfrom theManchester RoyalInfirmary.Perimortemcaesareansection.EmergMedJ 2002;19:324–5.
- AllenMC,DonohuePK,DusmanAE.Thelimitofviability–neonataloutcome ofinfantsbornat 22to25weeks’gestation.NEnglJMed1993;329:1597–601.
- MooreC,PromesSB.Ultrasoundinpregnancy.EmergMedClinNorthAm 2004;22:697–722.
- RittenbergerJC,KellyE,JangD,GreerK,HeffnerA.Successfuloutcomeutilizing hypothermia aftercardiacarrestinpregnancy:acasereport.CritCareMed 2008;36:1354–6.
- Natale A, Davidson T, Geiger MJ, Newby K. Implantable cardioverterdefibrillators and pregnancy:asafecombination?Circulation1997;96: 2808–12.
- SiassakosD,CroftsJF,WinterC,WeinerCP,DraycottTJ.Theactivecomponents ofeffective traininginobstetricemergencies.BJOG2009;116:1028–32.
- Siassakos D, Bristowe K, Draycott TJ, et al. Clinical efficiency in a simulated emergency and relationshiptoteambehaviours:amultisitecross-sectional study.BJOG2011;118:596–607.
- McNally B, Robb R, Mehta M, et al. Out-of-Hospital Cardiac Arrest Surveillance – Cardiac Arrest Registry to Enhance Survival (CARES), United States, October 1, 2005–December 31, 2010.MMWRSurveillSumm2011;60:1–19.
- Chugh SS, Jui J, Gunson K, et al. Current burden of sudden cardiac death: multiple source surveillanceversusretrospectivedeathcertificate-basedreviewin alargeU.S.community.JAm CollCardiol2004;44:1268–75.
- Churpek MM, Yuen TC, Winslow C, Hall J, Edelson DP. Differences in vital signs between elderlyandnonelderlypatientspriortowardcardiacarrest.CritCare Med2015;43:816–22.
- VanHoeyweghenRJ,BossaertLL,MullieA,etal.Survivalafterout-of-hospital cardiacarrestin elderlypatients.BelgianCerebralResuscitationStudyGroup. AnnEmergMed1992;21:1179–84.
- TungP,AlbertCM.Causesandpreventionofsuddencardiacdeathintheelderly. NatRevCardiol 2013;10:135–42.
- Teodorescu C, Reinier K, Dervan C, et al. Factors associated with pulseless electric activity versus ventricular fibrillation: the Oregon sudden unexpected death study. Circulation 2010;122:2116–22.
- Winther-Jensen M, Pellis T, Kuiper M, et al. Mortality and neurological outcome in the elderly after target temperature management for out-of-hospital cardiac arrest. Resuscitation 2015;91:92–8.
- LamantiaMA,StewartPW,Platts-MillsTF,etal.Predictivevalueofinitialtriage vitalsignsfor criticallyillolderadults.WestJEmergMed2013;14:453–60.
- NasaP,JunejaD,SinghO.Severesepsisandsepticshockintheelderly:an overview.WorldJCrit CareMed2012;1:23–30.
- Tresch DD. Management of the older patient with acute myocardial infarction: difference in clinicalpresentationsbetweenolderandyoungerpatients.JAm GeriatrSoc1998;46:1157–62.
- Tresch DD. Signs and symptoms of heart failure in elderly patients. Am J Geriatr Cardiol 1996;5:27–33.
- Gardin JM, Arnold AM, Bild DE, et al. Left ventricular diastolic filling in the elderly: the cardiovascularhealthstudy.AmJCardiol1998;82:345–51.
- PriebeHJ.Theagedcardiovascularriskpatient.BrJAnaesth2000;85: 763–78.
- Hasegawa K, Hagiwara Y, Imamura T, et al. Increased incidence of hypotension in elderly patients who underwent emergency airway management: an analysis of a multi-centre prospectiveobservationalstudy.IntJEmergMed 2013;6:12.
- MontamatSC,CusackBJ,VestalRE.Managementofdrugtherapyintheelderly. NEnglJMed 1989;321:303–9.
- Black CJ, Busuttil A, Robertson C. Chest wall injuries following cardiopulmonary resuscitation.Resuscitation2004;63:339–43.
- Krischer JP, Fine EG, Davis JH, Nagel EL. Complications of cardiac resuscitation. Chest 1987;92:287–91.
- Kashiwagi Y, Sasakawa T, Tampo A, et al. Computed tomography findings of complications resultingfromcardiopulmonaryresuscitation.Resuscitation 2015;88:86–91.
- GrimaldiD,DumasF,PerierMC,etal.Short-andlong-termoutcomeinelderly patientsafterout-of-hospitalcardiacarrest:acohortstudy.CritCareMed 2014;42:2350–7.
- NolanJP,SoarJ,SmithGB,etal.Incidenceandoutcomeofin-hospitalcardiacarrestintheUnited KingdomNationalCardiacArrestAudit.Resuscitation 2014;85:987–92.
- Deasy C, Bray JE, Smith K, et al. Out-of-hospital cardiac arrests in the older age groups in Melbourne,Australia.Resuscitation2011;82:398–403.
- Chan PS, Nallamothu BK, Krumholz HM, et al. Long-term outcomes in elderly survivors of in-hospitalcardiacarrest.NEnglJMed2013;368:1019–26.
- vandeGlindEM,vanMunsterBC,vandeWeteringFT,vanDeldenJJ,Scholten RJ,HooftL.Pre-arrest predictors of survival after resuscitation from outof-hospital cardiac arrest in the elderly systematicreview.BMCGeriatr 2013;13:68.
- MenonPR,EhlenbachWJ,FordDW,StapletonRD.Multiplein-hospitalresuscitationeffortsin theelderly.CritCareMed2014;42:108–17.
- BunchTJ,WhiteRD,KhanAH,PackerDL.Impactofageonlong-termsurvivalandqualityoflife followingout-of-hospitalcardiacarrest.CritCareMed 2004;32:963–7.
- Boyd K, Teres D, Rapoport J, Lemeshow S. The relationship between age and the use of DNR ordersincriticalcarepatients.Evidenceforagediscrimination. ArchInternMed1996;156:1821–6.
- Schwenzer KJ, Smith WT, Durbin Jr CG. Selective application of cardiopulmonary resuscitationimprovessurvivalrates.AnesthAnalg1993;76: 478–84.
- Seder DB, Patel N, McPherson J, et al. Geriatric experience following cardiac arrest at six interventional cardiology centers in the United States 2006–2011: interplay of age, do-not-resuscitateorder,andoutcomes.CritCareMed 2014;42:289–95.
