CLINICAL GUIDELINES
Danish Guidelines 2023 for Percutaneous Dilatational Tracheostomy in the Intensive Care Unit
Christina Sørensen, David Buck, Jonathan White, Pia Lawson-Smith
This 2023 revised edition of the 2015 edition has been approved by the Danish Society of Anesthesiology and Intensive Care Medicine (DASAIM) and the Danish Society of Intensive Care Medicine (DSIT) on 26th January 2023
Correspondence: Jonathan White, Consultant Intensive Care Unit 4131, Rigshospital, Copenhagen.
Jonathan.oliver.white@regionh.dk
Summary:
Percutaneous dilatational tracheostomy is a common procedure in intensive care. This updated Danish national guideline describes indications, contraindications and complications, and gives recommendations for timing, anaesthesia, and technique, use of fiberoptic bronchoscopy and ultrasound guidance, as well as decannulation strategy, training, and education.
Limitation: Applicable only for patients aged ≥ 15 years
Last literature review: January 2015
Next update: January 2027
List of abbreviations:
ICU = intensive care unit
PDT = percutaneous dilatational tracheostomy
ST = surgical tracheostomy
RCT = randomized controlled trial
PICO = population, intervention, comparator, outcome
DASAIM = Danish Society of Anesthesiology and Intensive Care Medicine
DSIT = Danish Society of Intensive Care Medicine
OR = operating room
INTRODUCTION
Tracheostomy is one of the most common surgical procedures performed in critically ill adults on intensive care units in patients requiring long term ventilation. The percutaneous procedure has been practiced and refined over the last 35 years, after having been described by Ciagla in 1985[1],, and has become the preferred procedure in the ICU.
CONTRIBUTORS, METHODS, SEARCH STRATEGY, AND LEVEL OF EVIDENCE
Contributors
A group of Danish ICU doctors with special interest and expertise in PDT was constituted.
Research questions
Where possible formal research questions were formulated, all concerning tracheostomy in mechanically ventilated adult critically ill patients in the ICU:
Which indications, contraindications, and complications should be appreciated? What is the optimal timing of tracheostomy?
Should PDT be preferred as standard method over ST?
Should fiberscopic guidance be used? Should ultrasound guidance be used?
Which form of anaesthesia is preferable?
How is training and education for PDT best organized?
PICO questions
Subtopics and PICO questions[2] were formulated and delegated to individual authors within the group, who in turnhanded in a draft for internal peer review.
Population:
Ventilated adult critically ill patients in the ICU
Intervention: PDT
Comparator: Any
Outcome: Mortality, morbidity, bleeding, pneumonia, length of mechanical ventilation, length of stay and serious adverse advents
Search strategy
PubMed and Cochrane Library were searched for literature. In addition, we hand-searched reference lists of relevant publications. No study designs were per se excluded but emphasis was put on RCTs and well performed recent metaanalyses.
Inclusion criteria
Adult critically ill patients in the ICU undergoingmechanical ventilation.
Exclusion criteria
Age less than 15 years. Studies conducted in a non-ICU setting
Validation and grading of evidence
We evaluated trial data using the GRADE approach (www.gradeworkinggroup.org). The GRADE system does not grade the quality of single studies but sequentially assesses the quality of evidence from the best available data for the outcomes of interest followed by assessment of the balance between benefits versus risks, burden, and cost. Literature identified by the search strategy was considered to represent the best-quality evidence. The quality of the evidence was quantified (high, moderate, low or very low) and potentially downgraded in the domains 1) risk of bias, 2) inconsistency of results, 3) indirectness of the evidence, 4) imprecision of results, and 5) other considerations including suspicion of publication bias, and was downgraded based on the number of domains with concerns (Table 1).
Recommendations
The recommendations were agreed upon in the group, and if total agreement could not be obtained, the group voted; 3/4 of the votes were needed to issue a strong recommendation. Strong recommendations (marked 1) were given the wording ‘we recommend’ and weak recommendations (2) ‘we suggest’. The level of evidence was graded high (marked A), moderate (B), low (C)or very low (D) based on the number of domains that were downgraded in adherence to GRADE.
Table 1. Rating the quality of evidence according to GRADE.
Source: Balshem et al.[3].
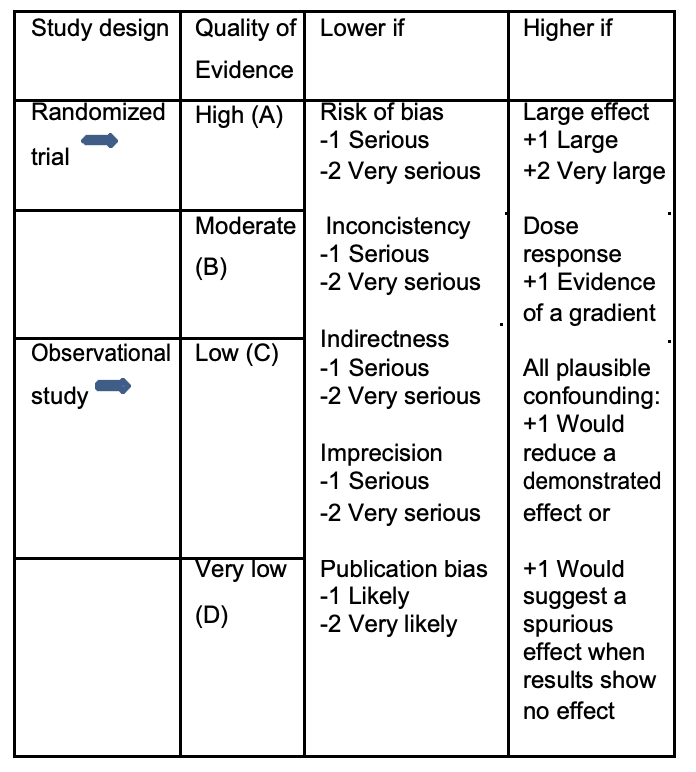
Table 2. Key recommendations


PDT – INDICATIONS AND CONTRAINDICATIONS
Indications for PDT:
Prolonged/expected prolonged mechanical ventilation Facilitate weaning from mechanical ventilation Airway protection against pulmonary aspiration (e.g. laryngeal incompetence due to critical illness, polyneuropathy, or bulbar dysfunction) Prolonged need for intratracheal suction Upper airway obstruction (e.g. tumor, bilateral recurrens paresis) Trauma or infection in oral cavity, pharynx or larynx. Minimisation of sedation Improved patient comfort.
Contraindications against PDT:
Informed refusal to the procedure Unstable fractures of the cervical spine Severe local infection of the anterior neck Uncontrollable coagulopathy.
Relative contraindications:
Controlled local infection Coagulopathy High PEEP or FiO2 requirements Difficult anatomy (e.g. morbid obesity, short thick neck, reduced neck extension, excessive goiter, tracheal deviation) Proximity to extensive burns or surgical wounds Elevated intracranial pressure Haemodynamic instability Previous radiotherapy to the neck.
No randomized, controlled trials concerning indications or contraindications for PDT were found. In experienced hands, PDT seems to be a safe procedure. The risk/benefit and timing of PDT should be evaluated on an individual patient basis. Usually PDT is an elective procedure, and all reversible risk factors (e.g. severe coagulopathy or excessive PEEP/FiO2 requirements) should be corrected in advance. The number of relative contraindications to PDT declines with increasing operator experience. A case series with 207 patients showed that PDT can even be performed safely as an emergency procedure by experienced clinicians[4]. Also PDT has been performed safely at high PEEP/FiO2 requirements[5]. and with few complications in spite of coagulopathy[6] and even though patients with severe thrombocytopenia (platelet count <50.000/microL) has a five-fold risk of persistent stomal bleeding PDT can be performed safely after administration of platelets in experienced hands 7 . With respect to patient receiving antiplatelet therapy and anticoagulants, the evidence is sparse but PDT seems to be safe even though the risk of bleeding is increased [8] [9]. Retrospective data has indicated that obese patients (BMI > 27,5) have a 5 times higher risk of severe perioperative complications with respect to PDT than normal-weight patients [10]. Whereas a larger cohort study found PDT to be just as safe as surgical tracheostomy (ST) in 143 morbidly obese patients (BMI ≥ 35) [11] with an overall complication rate comparable to non-obese patients (5,6% vs 4% ) [12]. Some small studies and case reports have reported fewer laryngeal complications with tracheostomy as compared with prolonged translaryngeal intubation [13]. Potential advantages with tracheostomy compared to prolonged translaryngeal intubation: Less sedation needed for tube acceptance Higher patient comfort (mobilisation, oral hygiene, phonation) Reduced risk of laryngeal damage in long-term intubation Reduced airway resistance and respiratory work More efficient cough Faster weaning from mechanical ventilation Shorter ICU-stay.
Potential advantages with tracheostomy compared to prolonged translaryngeal intubation:
Less sedation needed for tube acceptance
Higher patient comfort (mobilisation, oral hygiene, phonation)
Reduced risk of laryngeal damage in long-term intubation
Reduced airway resistance and respiratory work
More efficient cough
Faster weaning from mechanical ventilation
Shorter ICU-stay
TIMING OF TRACHEOSTOMY IN THE CRITICALLY ILL – EARLY VERSUS LATE?
Population: Mechanically ventilated adult critically ill patients in the ICU Intervention: Early tracheostomy
Comparator: Late or no tracheostomy
Outcome: Mortality, pneumonia, duration of mechanical ventilation and ICU or hospital stay
Recommendation: In prolonged mechanical ventilation, we suggest that optimal timing of tracheostomy be determined on an individual patient basis (2B).
The definition of what constitutes early vs. late tracheostomy has not been categorically defined and there is hence no ‘gold standard’. The latest Cochrane review (2012) defined early as < 10 days and late > 10 days, trial authors have been free to use any arbitrary definitions.
All RCTs so far have been underpowered to detect a possible small yet clinically relevant benefit of early timing of this widespread procedure in the ICU. A host of meta-analyses [14] [15] [16] [17] [18] [19] [20] [21] [22] have returned non-significant results on outcomes such as mortality, pneumonia, duration of mechanical ventilation, and length of intensive care or hospital stay and the two most recent have given conflicting results [23]. On this background, we find insufficient evidence to support a firm recommendation of early versus late tracheostomy in routine clinical practice. Optimal timing of tracheostomy should be determined individually with daily clinical assessment.
PDT VS. ST
Population: Mechanically ventilated adult critically ill patients in the ICU
Subject: PDT vs ST
Outcome: Early and late complications. Resource utilization.
Recommendation: We recommend bedside PDT as the standard method for tracheostomy in intensive care patients (1B)
– bedside PDT is logistically simpler and has fewer or equally few complications compared to ST (1A)
– bedside PDT is less expensive than ST in the operating room (1B)5. PDT VS. ST Population:
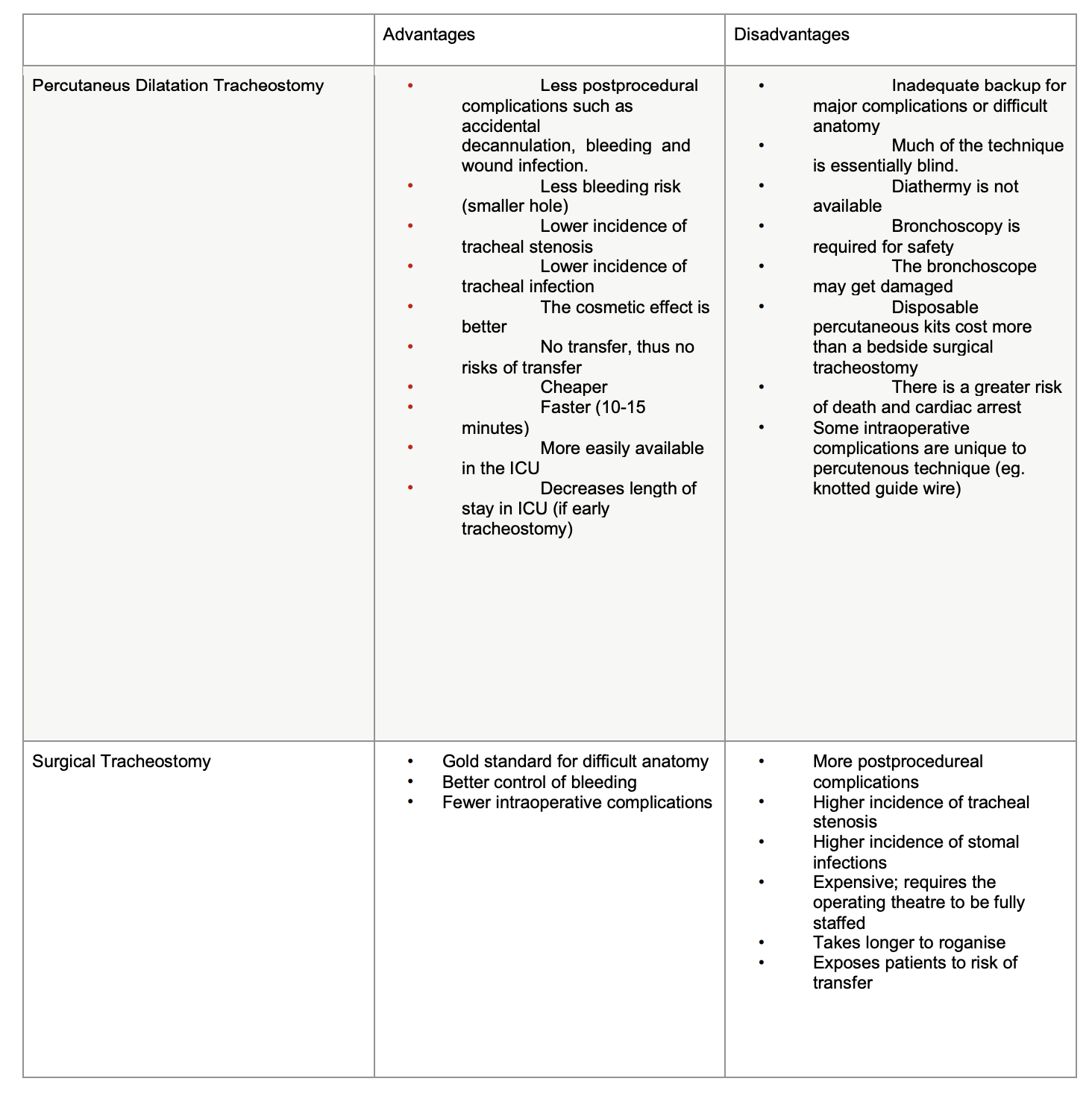
Table 3.
We recommend that surgical tracheostomy in the operating room remains the back-up method in difficult cases (ungraded best clinical practice) We recommend that surgical tracheostomy in the operating room
remains the back-up method in difficult cases (ungraded best clinical practice) 24
When :
– Anatomical landmarks are difficult to palpate
– Blood vessels at the site of insertion
– Malignancy at site of insertion
– If emergency tracheostomy is required
Background:
In controlled studies, clinically important complications are infrequent following both PDT or ST. Most serious or fatal complications such as
uncontrollable bleeding or irreversible loss of airway have only been published in case reports. Table 3 shows the advantages and
disadvantages in PDT and ST.
Early complications
Johnson-Obaseki et al (2016) 25 concluded that with regard to rates of mortality, intraoperative hemorrhage, and postoperative hemorrhage
there was no statistically significant difference between the two techniques The same authors found that the post-procedure infection rate
was lower with the percutaneous technique. This has been confirmed by Raymond et al 26
.
Resource utilization
The above mentioned meta-analysis from Johnson-Obaseki et al 25 found that the procedure time was faster for the percutaneous technique.
as well as requiring much less personnel.
Late complications
The risk of tracheal stenosis was found to be higher for surgical tracheostomy,though only as a trend, rather than a firm association 27
.
A meta-analysis from 2006 28 of 17 RCTs including 1212 patients found a significantly reduced wound infection rate of 2,3 % after bedside
PDT versus 10,7 % following surgical tracheostomy either bedside or in the OR. A possible cause is the minimally invasive surgical
technique with PDT.
Bleeding requiring transfusion or subsequent surgical haemostasis was seen in 5-6 % in both groups. A subgroup analysis of bedside PDT
versus surgical tracheostomy in the OR revealed a significantly lower risk of bleeding and lower mortality with bedside PDT. This finding
could reflect the risk of intrahospital transport of a critically ill patient.
Also the financial cost of bedside PDT is lower than that of surgical tracheostomy in the OR 29
.
The most significant study 30 in the above-mentioned meta- analysis randomized 200 ICU patients to either bedside surgical or percutaneous
tracheostomy. No significant difference was found in the combined primary endpoint (bleeding, infection, pneumothorax, accidental
decannulation, other major operative complication, or death). The total complication rate was low: 3,5 %. However, there were fewer stomal
infections in the PDT group at day 7. Also time from randomization to tracheostom [24] When:
- Anatomical landmarks are difficult to palpate
- Blood vessels at the site of insertion
- Malignancy at site of insertion
- If emergency tracheostomy is required
Background:
In controlled studies, clinically important complications are infrequent following both PDT or ST. Most serious or fatal complications such as uncontrollable bleeding or irreversible loss of airway have only been published in case reports. Table 3 shows the advantages and disadvantages in PDT and ST.
Early complications
Johnson-Obaseki et al (2016) [25] concluded that with regard to rates of mortality, intraoperative hemorrhage, and postoperative hemorrhage there was no statistically significant difference between the two techniques The same authors found that the post-procedure infection rate was lower with the percutaneous technique. This has been confirmed by Raymond et al. [26].
Resource utilization
The above mentioned meta-analysis from Johnson-Obaseki et al. [25] found that the procedure time was faster for the percutaneous technique. as well as requiring much less personnel.
Late complications
The risk of tracheal stenosis was found to be higher for surgical tracheostomy,though only as a trend, rather than a firm association [27]. A meta-analysis from 2006 [28] of 17 RCTs including 1212 patients found a significantly reduced wound infection rate of 2,3 % after bedside PDT versus 10,7 % following surgical tracheostomy either bedside or in the OR. A possible cause is the minimally invasive surgical technique with PDT.
Bleeding requiring transfusion or subsequent surgical haemostasis was seen in 5-6 % in both groups. A subgroup analysis of bedside PDT versus surgical tracheostomy in the OR revealed a significantly lower risk of bleeding and lower mortality with bedside PDT. This finding could reflect the risk of intrahospital transport of a critically ill patient.
Also the financial cost of bedside PDT is lower than that of surgical tracheostomy in the OR [29].
The most significant study [30] in the above-mentioned meta- analysis randomized 200 ICU patients to either bedside surgical or percutaneous tracheostomy. No significant difference was found in the combined primary endpoint (bleeding, infection, pneumothorax, accidental decannulation, other major operative complication, or death). The total complication rate was low: 3,5 %. However, there were fewer stomal infections in the PDT group at day 7. Also time from randomization to tracheostomy was shorter in the PDT group. The latter could reflect the logistical advantage of the intensivists themselves performing the procedure.
ANAESTHESIA FOR PDT
Recommendation:
We suggest that anaesthesia for PDT should consist routinelyof intravenous general anaesthesia and neuromuscular blockade (2D)
We suggest that PDT can also be safely carried out in local analgesia (2D).
We suggest the laryngeal mask airway as a safe alternative toretracting an endotracheal tube (2B).
Usual fasting rules are applicable.
Prepare for a difficult airway(ungraded)
Randomised clinical studies of anesthesia for PDT were not identified, so this recommendation relies primarily on expert opinion and case reports. Sedation to tube tolerance is not sufficient for surgical anesthesia Neuromuscular blockade optimises surgical conditions and facilitates controlled ventilation. Inhalational anaesthesia is avoided due to the inherent gas leakage during the procedue. In this procedure, managing the airway is the anaesthesiologist’s greatest challenge [31].
To facilitate the surgical procedure, the upper part of the back is elevated and the neck hyperextended which makes laryngoscopy more difficult. Prior prolonged intubation constitutes a risk for airway oedema [32]. The orotracheal tube can be retracted under direct laryngoscopy until the cuff is just distal to the vocalcords, before the trachea is punctured [33] Still there is a risk that the introducing needle hits the tracheal tube if the tip of the tubeis not proximal to the puncture site.
An alternative is to extubate the patient and insert a laryngeal mask, where the risks are pulmonary aspiration, air leakage and compromised ventilation. However one randomized clinical trial concludes that the laryngeal mask airway has significant advantages over withdrawing an endotracheal tube [34].
The exact choice of method depends on clinical evaluation and personal preference. Equipment for managing the difficult airway should be available.
PDT: TECHNIQUE AND PROCEDURE
Recommendation, PDT technique:
We do not recommend any specific percutaneous technique. (grade 1B)
We suggest the choice of a percutaneous technique to be based on the operator´s experience, clinical judgment, and local practice. (grade2D)
Several commercial kits are available for PDT. Raimondi et al. c in 2017 a review of comparative studies regarding different modalities of PDT. All included studies were randomized trials and compared different techniques;
PDT with multiple dilatators
PDT with forceps
PDT with single dilatator
Rotational PDT Translaryngeal PDT
PDT with a balloon
There is no evidence to recommend one PDT technique over another. The method should be selected on the basis of clinical criteria, experience, availability and local practice.
The following suggestions for PDT procedure are based on expert opinions and rules of thumb. (ungraded).
Staff:
We recommend that two doctors should participate, one of whom should be experienced in the technique of PDT. (1D)
Preparation:
If the patient has the capacity to give consent then this must be obtained directly from the patient.
If the patient can not give consent then the patient’s next of kin are informed (if possible).
If the patient has appointed a power of attorney then they should give consent to the procedure
Anti-coagulation should be paused according to institutional practice.
Instruments:
PDT-kit.
Tracheostomy tube Laryngoscope, intubation tray and equipment for difficult airway management should be immediately available. Flexible bronchoscope (preferably a video bronchoscope, since it allows all personal in the room to visualize the positioning of the oral endotracheal tube) and a bronchoscope attachment for the ventilator.
Optional: ultrasound machine.
The procedure itself:
Patient in supine position. Placement of a shoulder roll under the scapula for optimal presentation of anterior neck anatomy. Identify anatomical landmarks; thyroid cartilage, cricoid cartilage and tracheal rings are palpated. The optimal site for tracheostomy is under the cricoid cartilage between the second and third tracheal ring. More proximal placement increases the risk of tracheal stenosis, whereas a more distal placement increases the risk of erosion of the great vessels in the mediastinum.
Palpate the neck to identify the pulse of any arteries in the area of planned incision. The choice of tracheostomy site can be guided with flexible bronchoscopy (light through the anterior tracheal wall) and/or ultrasound. Withdrawal of the tracheal tube under bronchoscopic guidance until the cuff is placed just under the vocal cords.
Antiseptic and sterile preparation according to institutional guidelines.
Local analgesia with a local anaesthetic containing adrenalin (to reduce bleeding) from skin to trachea.
Skin incision: 8-12 mm horizontal incision at the chosen level. The incision must be as short as possible to reduce risk of bleeding and infection and to provide a tight-fitting stoma.
Introduction of guidewire: The cuff of the tracheal tube is deflated, the trachea is punctured in the midline, and the guidewire is introduced. Broncosopic confirmation of intra-tracheal placement and visualization of distal guidewire placement.
Stomal dilatation with one or more dilators, possibly with the use of a dilating forceps. Direct visualization of intra-tracheal placement (bronchoscopic confirmation through oral tube).
Finally appropriate fixation according to institutional practice to avoid cannula displacement.
Complications:
Loss of airway
A new airway must be established expeditiously by
- creating the tracheostomy
- or replacing the endotracheal tube
Perioperative bleeding:
– Minor bleeding (no transfusion requirement):
- manual compression.
- subcutaneous infiltration with adrenaline containing local analgesic circumferentially to the tracheal stoma.
- compress soaked with adrenalin-solution (1 mg adrenalin, 4 ml sterile water) wrapped around the tube between the flange and the skin or with locally applied tranexamic acid.
– Major bleeding (transfusion requirement or continued bleeding despite the above measures)
- consult an ENT specialist (exploration, suture, cautery).
BRONCHOSCOPIC GUIDANCE:
We recommend bronchoscopic guidance for PDT (1D)
Background: No RCTs of PDT with bronchoscopic guidance versus no bronchoscopic guidance were identified. A systematic review26 and a meta-analysis [35] found no evidence of use of bronchoscopic guidance to decrease the number of complications. The primarily rationale for using bronchoscopy during PDT is
- Correct tracheostomy site (midline placement, level at tracheal rings, light at the anterior tracheal wall).
- Intra-tracheal guidewire placement.
- Intratracheal dilator placement without tracheal damage.
- Position of tracheal cannula postprocedurally
ULTRASOUND GUIDANCE:
We suggest the use of Ultrasound prior to PDT to identify anatomical structures (2C) Background:
A systematic review by Gobatti compared ultrasound guided PDT to bronchoscopic and landmark-guided PDT. They found no difference in complication rates. Neither did Raimondi [26]
The prior rationale for using Ultrasound before PDT is
- Evaluation of the anatomy of major vessels and the thyroid gland in relation to tracheostomy site or small vessels anterior to the tracheostomy site
- the position of the tracheal rings and the midline (even in obese patients 9.ULTRASOUND GUIDANCE :
We suggest the use of Ultrasound prior to PDT to identify anatomical structures (2C)
Background:
A systematic review by Gobatti compared ultrasound guided PDT to bronchoscopic and landmark-guided PDT. They found no
difference in complication rates. Neither did Raimondi 26
The prior rationale for using Ultrasound before PDT is
– Evaluation of the anatomy of major vessels and the thyroid gland in relation to tracheostomy site or small vessels anterior to
the tracheostomy site
– the position of the tracheal rings and the midline (even in obese patients [36])
– Gauging depth of subcutaneous tissue
– Improving puncture accuracy
There is so far insufficient evidence to make recommendations of routine use of ultrasound before PDT to reduce complications. - Gauging depth of subcutaneous tissue
- Improving puncture accuracy
There is so far insufficient evidence to make recommendations of routine use of ultrasound before PDT to reduce complications.
PATIENT SAFETY
Recommendation: In intensive care units caring for tracheostomized patients:
- We recommend that the Surgical Safety Check-list, as developed by WHO, and with local modifications, be
- routinely applied to the surgical procedure of PDT (1B) [37]
- We recommend capnography should be used in cases of suspected tracheal tubedisplacement (1D)
- We suggest that all clinical staff who work in ICU should be trained in interpretation ofcapnography (2D)
- We recommend the presence of a difficult airway trolley in close proximity to the unit (1D)
- We suggest the establishment of an algorithmto be used in the clinical scenario where there is suspicion of a
displaced tracheostomy (2D) - We suggest that all ICU doctors receive ongoing training in the use of supraglottic devices and are familiar in the techniques ofadvanced airway management (2D)
Background: Considering the results of the national audit project(NAP4) from the United Kingdom it seems prudent to implementtheabove measures [Citation].
DECANNULATION
Recommendations: (all ungraded best clinical practice)
The patient should be decannulated as soon as possible when
- Effective coughing
- Effective swallowing
- Inspiratory fraction of oxygen is reasonably low
- Suctioning is rarely needed
- Succesfull prolonged spontaneous breathing trial on a capped tracheostomy
- The airway is patent
At discharge from ICU to the general ward with a tracheal cannula in place:
- The tracheal cannula should be without cuff (to avoid risk of total airway occlusion) unless the ward is specialized in care of patients with cuffed tracheal cannulas.
- An individual plan for tracheostomy management and decannulation should be presented.
Background: Recommendations about decannulation suffer from lack of solid evidence and are largely based on expert opinions. Systematic reviews on the subject have identified the paucity of evidence and the need for [38] evidenced based decannulation protocols. They found that the decannulation is more often individualized than protocolized. They found some objective criteria to use in the clinical judgement of decannulation, but the criteria still need to be validated in daily practice.
A prospective observational study showed that discharge from ICU to the ward with a tracheal cannula in place was an independent risk factor for mortality, especially in case of a high BMI [39]. A survey in Denmark revealed inadequate post-ICU follow-up in nondecannulated patients on the ward [40]. An intensivist led, post ICU tracheostomy follow-up team has been associated with earlier discharge from hospital [41].
TRAINING AND EDUCATION
To minimise complications, PDT should be performed by doctors able to maintain their routine in this procedure, typically at a specialist level in intensive care medicine. In one study complication rates among residents performing PDT was found to be higher during their first five procedures than later [42](1).
Training a procedure, in this case PDT, involves both knowledge (indications, contraindications, complications), practical management (preparation, dexterity, technique) as well as communication and teamwork (consent, modesty, knowing when to call for senior assistance) .
When a colleague is training a procedure, the following steps are suggested (3):
- Demonstration: The supervisor demonstrates the procedure at a normal pace, but without comments.
- Deconstruction: The supervisor demonstrates and simultaneously describes the steps of the procedure.
- Understanding: The supervisor demonstrates the steps of the procedure, but this time with the trainee talking the supervisor through the steps.
- Management: The trainee demonstrates and describes the steps of the procedure.
In this way the procedure is split into manageable steps, and the trainee is asked to describe each step. The repetition reinforces the learning process, and possible mistakes are corrected. Also, different learning styles are possible, because the trainee sees, hears, describes and performs the procedure.
We recommend that the supervisor and the trainee meet two times as a minimum to ensure that all 4 steps are carried out.
Step 1 is demonstrated on a patient or a teaching video.
Step 2-4 can be trained theoretically, but preferably on a mannequin, where promising results with producing low cost PDT simulators indicate potentials [43]. The steps are repeated until the supervisor finds the trainee ready to perform the procedure on a clinical patient under supervision (step 4). It is individually decided when the colleague is ready to perform PDT without supervision.
We recommend the following structure for every learning session
- Introduction: The trainee’s basic knowledge of PDT? Consider the placement of the trainee: Next to you or opposite? Left- or righthanded?
- Dialogue: Have you broken down the PDT procedure into clearly defined steps? Do you give positive feedback to the trainee? (”What went well?”, ”What would you do differently next time?”) Avoid too much talk. Often too many details are given.
- Conclusion: Can the colleague safely perform PDT? How will he or she continue the learning process? Take home messages.
References
- Ciaglia P, Firsching R, Syniec C. Elective Percutaneous Dilatational Tracheostomy. Chest. 1985;87(6):715-719. doi:10.1378/chest.87.6.715.
- 2. Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 2. Framing the question and deciding on important outcomes. J Clin Epidemiol. 2011;64(4):395-400. doi:10.1016/j.jclinepi.2010.09.012.
- Balshem H, Helfand M, Schünemann HJ, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64(4):401- 406. doi:10.1016/j.jclinepi.2010.07.015.
- Klein M, Agassi R, Shapira AR, Kaplan DM, Koiffman L, Weksler N. Can intensive care physicians safely perform percutaneous dilational tracheostomy? An analysis of 207 cases. Isr Med Assoc J IMAJ. 2007;9(10):717-719.
- 5. Beiderlinden M, Groeben H, Peters J. Safety of percutaneous dilational tracheostomy in patients ventilated with high positive endexpiratory pressure (PEEP). Intensive Care Med. 2003;29(6):944-948. doi:10.1007/s00134-003-1656-8.
- Pilarczyk K, Dusse F, Marggraf G, Schönfelder B, Jakob H. Percutaneous dilatational tracheostomy in patients with severe coagulopathy or thrombocytopenia. Crit Care. 2014;18(Suppl 1):P326. doi:10.1186/cc13516.
- Kluge S, Meyer A, Kühnelt P, Baumann HJ, Kreymann G. Percutaneous Tracheostomy Is Safe in Patients With Severe Thrombocytopenia. Chest. 2004;126(2):547-551. doi:10.1378/chest.126.2.547.
- Pasin L, Frati E, Cabrini L, et al. Percutaneous tracheostomy in patients on anticoagulants. Ann Card Anaesth. 2015;18(3):329-334. doi:10.4103/0971-9784.159802.
- Huang YH, Tseng CH, Chan MC, Lee BJ, Lin CH, Chang GC. Antiplatelet agents and anticoagulants increased the bleeding risk of bedside percutaneous dilational tracheostomy in critically ill patients. J Formos Med Assoc Taiwan Yi Zhi. 2020;119(7):1193-1200. doi:10.1016/j.jfma.2019.10.014.
- Byhahn C, Lischke V, Meininger D, Halbig S, Westphal K. Peri-operative complications during percutaneous tracheostomy in obese patients. Anaesthesia. 2005;60(1):12-15. doi:10.1111/j.1365-2044.2004.03707.x.
- Heyrosa MG, Melniczek DM, Rovito P, Nicholas GG. Percutaneous tracheostomy: a safe procedure in the morbidly obese. J Am Coll Surg. 2006;202(4):618-622. doi:10.1016/j.jamcollsurg.2005.12.009.
- Díaz-Regañón G, Miñambres E, Ruiz A, González-Herrera S, Holanda-Peña M, López-Espadas F. Safety and complications of percutaneous tracheostomy in a cohort of 800 mixed ICU patients. Anaesthesia. 2008;63(11):1198-1203. doi:10.1111/j.1365- 2044.2008.05606.x.
- Rumbak MJ, Newton M, Truncale T, Schwartz SW, Adams JW, Hazard PB. A prospective, randomized, study comparing early percutaneous dilational tracheotomy to prolonged translaryngeal intubation (delayed tracheotomy) in critically ill medical patients*: Crit Care Med. 2004;32(8):1689-1694. doi:10.1097/01.CCM.0000134835.05161.B6.
- Griffiths J, Barber VS, Morgan L, Young JD. Systematic review and meta-analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. BMJ. 2005;330(7502):1243. doi:10.1136/bmj.38467.485671.E0.
- Durbin CG, Perkins MP, Moores LK. Should tracheostomy be performed as early as 72 hours in patients requiring prolonged mechanical ventilation? Respir Care. 2010;55(1):76-87.
- Dunham CM, Ransom KJ. Assessment of early tracheostomy in trauma patients: a systematic review and meta-analysis. Am Surg. 2006;72(3):276-281. doi:10.1177/000313480607200316.
- Wang F, Wu Y, Bo L, et al. The timing of tracheotomy in critically ill patients undergoing mechanical ventilation: a systematic review and meta-analysis of randomized controlled trials. Chest. 2011;140(6):1456-1465. doi:10.1378/chest.11-2024.
- Gomes Silva BN, Andriolo RB, Saconato H, Atallah AN, Valente O. Early versus late tracheostomy for critically ill patients. Cochrane Database Syst Rev. 2012;(3):CD007271. doi:10.1002/14651858.CD007271.pub2.
- Huang H, Li Y, Ariani F, Chen X, Lin J. Timing of tracheostomy in critically ill patients: a meta-analysis. PloS One. 2014;9(3):e92981. doi:10.1371/journal.pone.0092981.
- Liu CC, Livingstone D, Dixon E, Dort JC. Early versus Late Tracheostomy: A Systematic Review and Meta-Analysis. Otolaryngol Neck Surg. 2015;152(2):219-227. doi:10.1177/0194599814561606.
- Hosokawa K, Nishimura M, Egi M, Vincent JL. Timing of tracheotomy in ICU patients: a systematic review of randomized controlled trials. Crit Care. 2015;19(1):424. doi:10.1186/s13054-015-1138-8.
- Andriolo BN, Andriolo RB, Saconato H, Atallah ÁN, Valente O. Early versus late tracheostomy for critically ill patients. Cochrane Emergency and Critical Care Group, ed. Cochrane Database Syst Rev. Published online January 12, 2015. doi:10.1002/14651858.CD007271.pub3.
- Siempos II, Ntaidou TK, Filippidis FT, Choi AMK. Effect of early versus late or no tracheostomy on mortality and pneumonia of critically ill patients receiving mechanical ventilation: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(2):150-158. doi:10.1016/S2213-2600(15)00007-7.
- Mehta C, Mehta Y. Percutaneous tracheostomy. Ann Card Anaesth. 2017;20(5):19. doi:10.4103/0971-9784.197793.
- Johnson-Obaseki S, Veljkovic A, Javidnia H. Complication rates of open surgical versus percutaneous tracheostomy in critically ill patients: Surgical Versus Percutaneous Tracheostomy. The Laryngoscope. 2016;126(11):2459-2467. doi:10.1002/lary.26019
- Raimondi N, Vial MR, Calleja J, et al. Evidence-based guidelines for the use of tracheostomy in critically ill patients. J Crit Care. 2017;38:304-318. doi:10.1016/j.jcrc.2016.10.009
- Dempsey GA, Morton B, Hammell C, Williams LT, Tudur Smith C, Jones T. Long-Term Outcome Following Tracheostomy in Critical Care: A Systematic Review*. Crit Care Med. 2016;44(3):617-628. doi:10.1097/CCM.0000000000001382.
- Delaney A, Bagshaw SM, Nalos M. Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: a systematic review and meta-analysis. Crit Care Lond Engl. 2006;10(2):R55. doi:10.1186/cc4887.
- Bacchetta MD, Girardi LN, Southard EJ, et al. Comparison of Open Versus Bedside Percutaneous Dilatational Tracheostomy in the Cardiothoracic Surgical Patient: Outcomes and Financial Analysis. Ann Thorac Surg. 2005;79(6):1879-1885. doi:10.1016/j.athoracsur.2004.10.042.
- Silvester W, Goldsmith D, Uchino S, et al. Percutaneous versus surgical tracheostomy: A randomized controlled study with long-term follow-up. Crit Care Med. 2006;34(8):2145-2152. doi:10.1097/01.CCM.0000229882.09677.FD.
- Bodenham PH. Percutaneous Tracheostomy. A Practical Handbook. Vol 2004. Greenwich Medical Media
- Lavery GG, McCloskey BV. The difficult airway in adult critical care. Crit Care Med. 2008;36(7):2163-2173. doi:10.1097/CCM.0b013e31817d7ae1.
- Schwann NM. Percutaneous dilational tracheostomy: anesthetic considerations for a growing trend. Anesth Analg. 1997;84(4):907- 911. doi:10.1097/00000539-199704000-00037.
- Linstedt U, Zenz M, Krull K, Häger D, Prengel AW. Laryngeal mask airway or endotracheal tube for percutaneous dilatational tracheostomy: a comparison of visibility of intratracheal structures. Anesth Analg. 2010;110(4):1076-1082. doi:10.1213/ANE.0b013e3181d27fb4.
- Iftikhar IH, Teng S, Schimmel M, Duran C, Sardi A, Islam S. A Network Comparative Meta-analysis of Percutaneous Dilatational Tracheostomies Using Anatomic Landmarks, Bronchoscopic, and Ultrasound Guidance Versus Open Surgical Tracheostomy. Lung. 2019;197(3):267-275. doi:10.1007/s00408-019-00230-7.
- Guinot PG, Zogheib E, Petiot S, et al. Ultrasound-guided percutaneous tracheostomy in critically ill obese patients. Crit Care Lond Engl. 2012;16(2):R40. doi:10.1186/cc11233.
- Haynes AB, Weiser TG, Berry WR, et al. A Surgical Safety Checklist to Reduce Morbidity and Mortality in a Global Population. N Engl J Med. 2009;360(5):491-499. doi:10.1056/NEJMsa0810119.
- Singh RK, Saran S, Baronia AK. The practice of tracheostomy decannulation—a systematic review. J Intensive Care. 2017;5(1):38. doi:10.1186/s40560-017-0234-z.
- Martinez GH, Fernandez R, Casado MS, et al. Tracheostomy tube in place at intensive care unit discharge is associated with increased ward mortality. Respir Care. 2009;54(12):1644-1652.
- Mondrup F, Skjelsager K, Madsen KR. Inadequate follow-up after tracheostomy and intensive care. Dan Med J. 2012;59(8):A4481.
- Tobin AE, Santamaria JD. An intensivist-led tracheostomy review team is associated with shorter decannulation time and length of stay: a prospective cohort study. Crit Care Lond Engl. 2008;12(2):R48. doi:10.1186/cc6864.
- Lake FR, Hamdorf JM. Teaching on the run tips 5: teaching a skill. Med J Aust. 2004;181(6):327-328. doi:10.5694/j.1326- 5377.2004.tb06301.x.
- Lake FR, Ryan G. Teaching on the run tips 3: planning a teaching episode. Med J Aust. 2004;180(12):643-644. doi:10.5694/j.1326- 5377.2004.tb06130.x.
